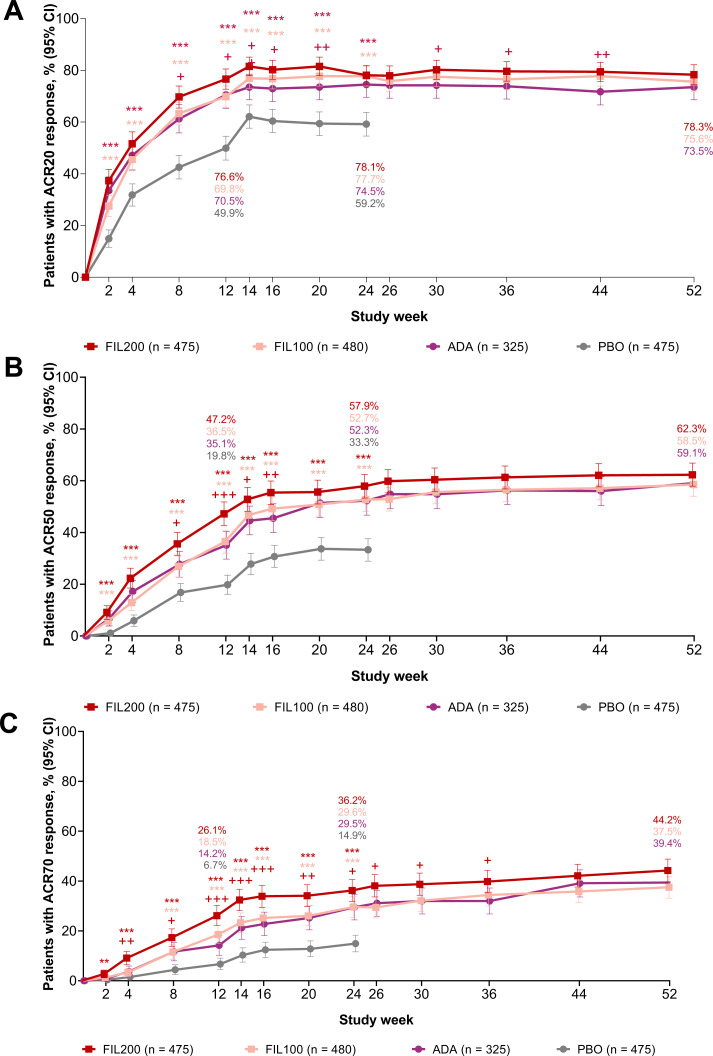
Figure 2.
Proportions of patients achieving (A) ACR20, (B) ACR50 and (C) ACR70 through week 52. Error bars show 95% CI. Additional statistical details are available in online supplemental table S3 and all response rates in online supplemental table S7. **p<0.01, ***p<0.001 versus PBO, not adjusted for multiplicity and should be considered exploratory except for ACR20 for FIL200 and FIL100 versus PBO at week 12. +p<0.05, ++p<0.01,+++p<0.001 versus ADA, not adjusted for multiplicity and should be considered exploratory. ACR20/50/70, 20%/50%/70% improvement from baseline by the American College of Rheumatology core criteria; ADA, adalimumab; FIL100, filgotinib 100 mg; FIL200, filgotinib 200 mg; PBO, placebo.