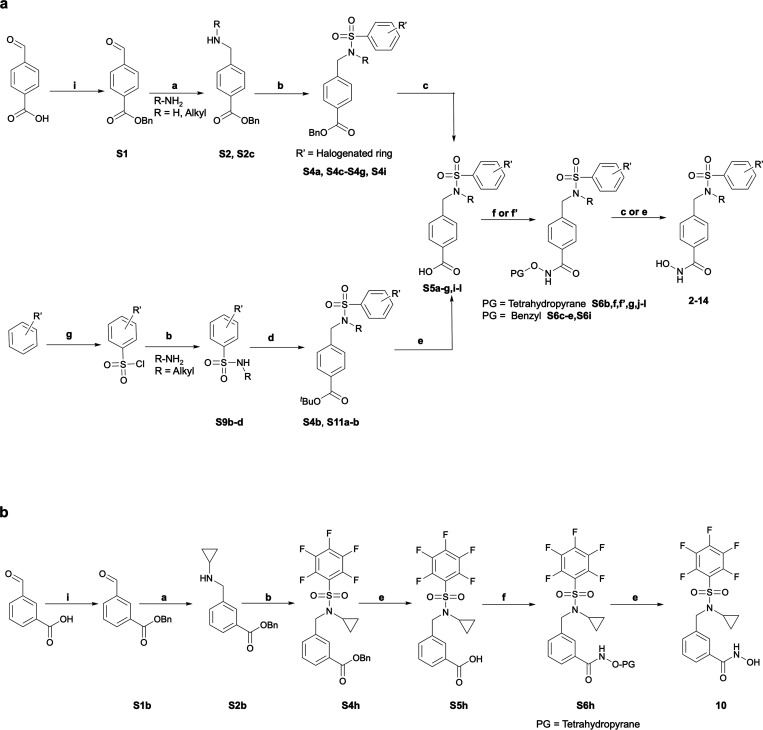
Scheme 1. Synthetic Conditions for Preparation of 2–14.
(a) Reagents and conditions to synthesize 2–9 and 11–14. (i) BnBr, Cs2CO3, DMF, RT, 24 h; (a) (i) R–NH2, AcOH, DCE, RT, 2 h; (ii) NaBH(OAc)3, RT, 16 h; (b) R′SO2Cl, Et3N, CH2Cl2, 3–16 h, RT; (c) H2, 10% Pd/C, THF/MeOH (2:1), RT, 16 h; (g) HSO3Cl, 3 h, 150°C; (b) RNH2, Et3N, CH2Cl2, RT, 3–16 h d) C12H15BrO2, Cs2CO3, DMF, RT, 24 h; e) 4M HCl/dioxane, 0°C-RT, 3 h; (f) (i) (COCl)2, THF, DMF, 0°C, 1 h; (ii) H2N-OTHP, iPr2NEt, THF, RT, 16 h; (f′) H2N-OBn, EDCI, HOBt, Et3N, DMF, RT, 16–24 h; (c) H2, 10% Pd/C, THF/MeOH (2:1), RT, 16–24 h; (e) 4M HCl/dioxane, 0°C-RT, 3 h. b Reagents and conditions to synthesize 10. (i) BnBr, Cs2CO3, DMF, RT, 24 h; a) (i) cPr-NH2, AcOH, DCE, RT, 2 h; (ii) NaBH(OAc)3, RT, 16 h; (b) PFBSCl, Et3N, CH2Cl2, 3–16 h, RT; (e) H2, 10% Pd/C, THF/MeOH (2:1), RT, 18 h; (f) (i) (COCl)2, THF, DMF, 0°C, 1 h; (ii) H2N-OTHP, iPr2NEt, THF, RT, 16 h; (e) H2, 10% Pd/C, THF/MeOH (2:1), RT, 6 h.