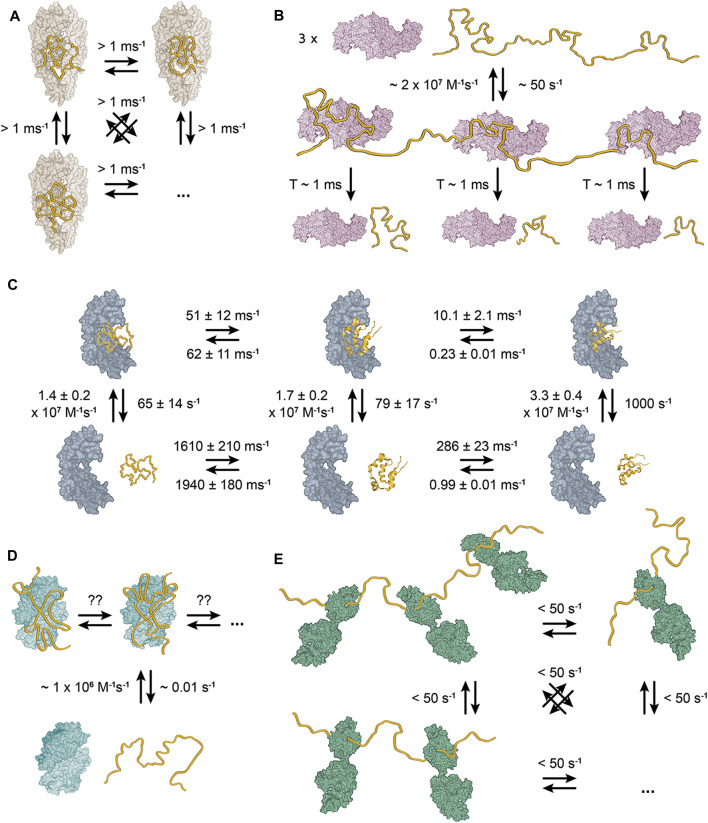
FIGURE 2.
Structural models of bacterial chaperone-client complexes reveal dynamic interactions. (A) The Skp-OMP complex. The client binds as compact, flexible ensemble, which interconverts between individual conformations within 1 ms (Burmann et al., 2013). (B) The trigger factor–PhoA complex. A single molecule of PhoA interacts with three molecules of TF with short-lived interaction lifetimes of ∼1 ms (Saio et al., 2014). The kinetics reveal that the complex is also globally short-lived with a dissociation rate of ∼50 s−1. (C) The Spy-Im7 complex. The chaperone Spy binds its client Im7 as a dynamic ensemble of diverse conformations (He et al., 2016; Stull et al., 2016; Horowitz et al., 2018). The representative unfolded state (on the left), folding intermediate state (in the middle) and native state (on the right) interconvert with ms rates. However, the rates are slower for Spy-bound Im7 than for free Im7. (D) The SecB-MBP complex. One molecule of the client binds one SecB tetramer (Huang et al., 2016). No symmetry breaking of the SecB tetramer is observed upon binding of the full-length clients, which indicates that the resulting complex must be dynamic with the client rearranging on SecB surface on a very fast timescale. (E) The DnaK–hTRF1 complex. DnaK binds the client in an ensemble of globally unfolded conformations at various stoichiometric ratios (Lee et al., 2015; Sekhar et al., 2016; Rosenzweig et al., 2017).