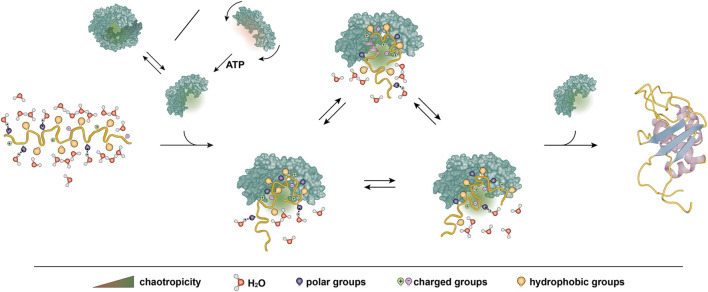
FIGURE 3.
Proposed model of chaotropicity underlying generic activity of chaperones. A client protein backbone is shown in yellow with different side chain groups and solvating water molecules as indicated. A chaperone is shown in green. The chaperone features a chaotropic pocket to bind and stabilize the protein, protecting it from premature hydrophobic collapse into a misfolded state and allowing it to explore the conformational space. The protein may fold into its native structure on the chaperone surface or upon release. Chaotropicity stems from the amino acids in the pocket surface and may thus be regulated for example by altering the accessibility of the pocket through dimerization or by ATP-regulated conformational changes.