Abstract
Fusion of cortical granules with oocyte plasma membrane is one of the most significant secretory events to prevent polyspermy during oocyte activation. Cortical granule exocytosis (CGE) is distinct from most other exocytosis because cortical granules are not renewed after secretion. However, it is thought to be mediated by SNARE complex, which mediates membrane fusion in other exocytoses. SNAREs proteins are divided into Q (glutamine)- and R (arginine)-SNAREs. Q-SNAREs include Syntaxins and SNAP25 family, and R-SNAREs include VAMPs family. In mouse oocytes, Syntaxin4 and SNAP23 have been involved in CGE; nevertheless, it is unknown if VAMP is required. Here, we demonstrated by RT-PCR and immunoblotting that VAMP1 and VAMP3 are expressed in mouse oocyte, and they localized in the cortical region of this cell. Using a functional assay to quantify CGE, we showed that tetanus toxin –which specifically cleavages VAMP1, VAMP2 or VAMP3– inhibited CGE suggesting that at least one VAMP was necessary. Function blocking assays demonstrated that only the microinjection of anti-VAMP1 or anti-VAMP3 antibodies abolished CGE in activated oocytes. These findings demonstrate that R-SNAREs sensitive to tetanus toxin, VAMP1 and VAMP3 –but not VAMP2-, are required for CGE and demonstrate that CGE is mediated by the SNARE complex.
Keywords: cortical granule exocytosis, VAMP, R-SNARE, cortical reaction, mouse oocyte
1. INTRODUCTION
The fusion of cortical granules (CG) with the oocyte plasma membrane (oolema) is one of the most significant events to prevent polyspermy. To guarantee the fertilization’s success and embryo development, a definitive block to polyspermy is necessary since oocyte’s fertilization by more than one sperm is embryonic lethal. Three postfertilization blocks to polyspermic fertilization have been described in mouse. The first two occur rapidly and their molecular basis remains unknown. The third, slow and definitive, correlates with the exocytosis of CG during Metaphase II (MII) oocyteś activation [10].
Cortical granule exocytosis (CGE), also known as the cortical reaction, is regulated by calcium and triggered by sperm after fertilization. CGE is distinct from most other regulated secretory vesicles because CG are not renewed after their fusion with the oocyte plasma membrane [20]. Despite being first described in mammals many decades ago [3], the molecular components and the molecular mechanism of CGE remain enigmatic.
Fusion of secretory granules and synaptic vesicles with the plasma membrane is driven by SNARE protein interactions. SNARE proteins are generally divided into two groups according to their cellular locations and functionalities: the v-SNAREs and the t-SNAREs [45;46]. The v-SNAREs are synaptic vesicle associated membrane proteins (VAMPs) that reside on the synaptic vesicles [50]. The t-SNAREs –represented by Syntaxin1 and synaptosomal-associated protein 25 kDa (SNAP25) and their variants– are cell presynaptic membrane proteins [6;37]. Both VAMP and Syntaxin have their C-terminal residues inserted in the membrane, whereas SNAP25 is anchored to the plasma membrane by palmitoylated cysteine residues in the central region [24;30;50;53]. Depending on which amino acid of the SNARE protein is involved in the SNARE core complex, SNAREs have been reclassified and divided into Q- and R-SNAREs [17]. Q-SNAREs include the t-SNARE proteins –the Syntaxin and SNAP25 family– as they contribute a glutamine (Q), whereas R-SNAREs include v-SNAREs –the VAMPs family– as they contribute an arginine (R) [17]. In neurotransmitter release, the Q- and R-SNAREs form a tight complex during the membrane fusion process, which is highly resistant to clostridium toxins [23;39]. This heterotrimeric complex is known as trans-SNARE complex because it pulls from two different membranes during membrane fusion: the synaptic vesicle and the plasma membrane. After membrane fusion, SNARE complex remains on the plasma membrane – cis-SNARE– and is disassembled by an accessory complex formed by alpha-SNAP and NSF [45;46]. This disassembling is necessary to allow a new round of membrane fusion during neurosecretion, a process in which synaptic vesicles are renewed or recycled.
The signal-transducing pathway accountable for CGE in mammals is not yet completely understood and is thought to be mediated by SNARE complexes, even when CG are not recycled after secretion. Thus, CGE only occurs once in the oocytés life since this secretory process is no further needed for the development of the embryo. Nevertheless, it is worth pointing out that the plasma membrane and its components may be retrieved during the compensatory endocytosis [21;44]. In porcine oocytes, it has been shown that the SNAREs Syntaxin2, SNAP23, VAMP1, and VAMP2 are expressed [51]. In mouse oocytes, only two proteins of the SNARE machinery have been identified, the Q-SNAREs SNAP23 and Syntaxin4. SNAP23 has been involved in cortical reaction [34] [34]; however the participation of Syntaxin4 in this secretory process has not been confirmed yet [28]. In addition, we have demonstrated that the alpha-SNAP/NSF complex is required for the cortical reaction and have proposed a working model [13]. However, whether CGE requires VAMP –also called R-SNARE– remains unknown in mouse oocytes.
VAMPs are a family with 9 predicted isoforms in humans [8]. So far seven VAMP isoforms have been identified: 1, 2, 3, 4, 5, 7 and 8, which have been characterized mainly in rodent models. Only the isoforms 1, 2, and 3 are involved in secretion and are the only ones sensitive to tetanus toxin. VAMP1 and VAMP2 are also known as synaptobrevin (syb) 1 and 2, respectively. VAMP1/syb1 is expressed in sensory neurons to regulate pain-peptide exocytosis [36]. VAMP2/syb2 is more abundant in the brain, where it regulates exocytosis of neurotransmitters from synaptic vesicles [42]. VAMP3, or cellubrevin, is not expressed in neurons and is thought to be the non-neuronal homologue of VAMP1 and VAMP2 in other secretory tissues [33].
The aim of this work was to investigate the expression and localization of the VAMPs isoforms involved exclusively in secretory processes such as VAMP1, VAMP2, and VAMP3 and to characterize, through functional assays, their participation in the cortical reaction. Here, we showed that VAMP1 and VAMP3, but not VAMP2, are expressed in mouse oocytes, and that both proteins participate in cortical granule exocytosis in mouse oocytes.
2. MATERIALS AND METHODS
2.1. Reagents
All chemicals, unless stated otherwise, were purchased from Sigma-Aldrich Chemical Inc. (St. Louis, USA).
2.2. Animals, superovulation and oocyte collection
Mouse oocytes were obtained from 8 to 12 weeks old CF-1 females, bred under controlled conditions of light and temperature. Immature oocytes (GV) were collected from intraperitoneally stimulated females with 10 IU of pregnant mare’s serum gonadotropin (PMSG; Syntex, Argentina) and 45–48h later the ovaries were punctured to obtain cumulus oocytes complexes (COC’s). The COC’s were collected in Earle’s balanced salt solution with 0.01% PVA, 0,001% Gentamycin, and 25 mM Hepes buffer, pH 7.3 (MEM/HEPES) supplemented with 2.5 μM Milrinone to prevent oocyte maturation. MII oocytes were collected from stimulated females with 10 IU of PMSG and 10 IU of human chorionic gonadotropin (hCG; Syntex, Argentina) at 48h and 13–17h before the collection, respectively. COC’s obtained from the oviductal ampullae were briefly incubated in 0.04% hyaluronidase to detach the cumulus cells from the oocytes. Until their use, the oocytes were maintained in M16 medium and covered with mineral oil in a humidified chamber (37°C, 5% CO2) for the shortest time possible. Controls and experimental oocytes were subjected to the same incubation times during different treatments. This study was carried out according to the recommendations described in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of the School of Medicine of the National University of Cuyo (Protocol approval 52/2015).
2.3. RNA extraction and reverse transcription-PCR
VAMP isoforms in GV oocytes and brain tissue were identified with the complementary DNA (cDNA) obtained from the retrotranscription of respectively mRNA. The cDNAs were amplified by Polimerase Chain Reaction (PCR). Total RNA was isolated from 50 to 100 pooled GV oocytes according to the standard protocol provided by the manufacturer of RNAqueous-Micro Kit (Ambion). Total RNA obtained from mouse brain was isolated with Trizol reagent (Invitrogen) following the manufactureŕs instructions. Whole isolated RNA from oocytes and 2 μg of brain RNA were used as template for reverse transcription into cDNA. cDNA was synthesized by incubating with mouse Moloney Leukaemia Virus (M-MLV) Reverse Transcriptase (Promega) and 1μg of Oligo dT (Biodynamics) during 1 h at 42 °C. To confirm the absence of contaminating residual DNA, the reaction was carried out without the M-MLV Reverse Transcriptase. The cDNA obtained from the reverse transcription was amplified by end-point PCR. PCR amplification was performed with cDNA obtained from 37 oocytes equivalents or 100 ng of brain cDNA, 1μM of each forward and reverse primer, 200 μM dNTPs (Promega); 5 μl Green GoTaq Reaction Buffer 5X (Promega), and 1 U of GoTaq DNA Polymerase (Promega) in a 25 μl volume reaction. The specific primers were used as follows: VAMP1 forward, 5’-CATGCGTGTGAATGTGGACAA-3’; VAMP1 reverse, 5’-GATGGCACAGATAGCTCCCAG-3’, PrimerBank ID: 29436399a1; VAMP2 forward, 5’-GCTGGATGACCGTGCAGAT-3’; VAMP2 reverse, 5’-GATGGCGCAGATCACTCCC-3’, PrimerBank ID: 6678551a1; VAMP3 forward, 5’-CCACTGGCAGTAATCGAAGAC-3’; VAMP3 reverse, 5’-ATCGCCCACATCTTGCAGTTC-3’, PrimerBank ID: 6678553a1. The expected size for VAMP1, 2, and 3 was 181, 130 and 220 bp, respectively. PCR negative control was carried out by excluding cDNA from the PCR mixture and substituting its volume for H2O. The reaction conditions for VAMP isoforms amplification were template denaturation and polymerase activation at 94 °C for 3 min, followed by 32 cycles of 94 °C denaturation for 1 min, 55,5 °C annealing for 45 s and 72 °C extension for 1.5 min, and a final extension at 72 °C for 5 min. The reactions were carried out using the Mastercycler Personal (Eppendorf) PCR thermal cycler. The PCR products were visualized on 2% agarose gels stained with SYBR safe DNA gel stain (Invitrogen) using ImageQuant LAS-4000 (Fujifilm).
2.4. Immunocytochemistry
The immunolocalization assays of VAMP isoforms were carried out in GV and MII oocytes. Zona pellucida was removed after a short incubation in acid Tyrode pH 2.2. Subsequently, oocytes were washed in MEM/HEPES and in Blocking Solution (BS) (3 mg/ml BSA, 100 mM glycine, 0.01% Tween 20). Cells were fixed for 1 h with paraformaldehide (PAF) 4% (Merck). Fixed oocytes were permeabilized in Triton X-100 0.1% during 15 min. Following blocking in BS, oocytes were incubated with primary antibodies overnight at 4 °C. Specific antibodies were used as follows, rabbit polyclonal anti-VAMP1 (1:10 dilution, Synaptic Systems, catalog number: 104 002); mouse monoclonal anti-VAMP2 (1:10 dilution, Synaptic Systems, catalog number: 104 211) and rabbit polyclonal anti-VAMP3 (1:10 dilution, Synaptic Systems, catalog number: 104 103). It was previously described that those antibodies are specific and they do not present cross-reactivity with the other two isoforms [51]. Once finished the incubation with the primary antibodies, cells were washed in BS and incubated with the secondary antibodies for 1 h at room temperature. The secondary antibodies used were DyLight 488 donkey anti-mouse (3 ng/μl, Jackson InmunoReasearch) and DyLight 488 donkey anti-rabbit (3ng/μl, JacksonInmunoReasearch). Oocytes were washed in BS and incubated during 30 min in 25 μg/ml lectin Lens Culinaris Agglutinin (LCA) conjugated to rhodamine to stain the CG. Finally, cells were mounted in Vectashield mounting medium (Vector Laboratories) inside a chamber under minimal compression. Nonspecific binding of the secondary antibody was determined by incubation without primary antibody. Confocal images were taken in the equatorial section of the cells and obtained using an Olympus confocal microscope. Imaging analysis was performed using ImageJ software (version 1.42l; NIH, MD).
2.5. Immunoblotting
Protein extract of 400 MII oocytes were separated on a 15% SDS PAGE gel, transferred to Immobilon-P, and immunoblotted according to our previous protocol [13]. The same primary antibodies described in the previous section were used as follows: anti-VAMP1, 1:500 dilution; anti-VAMP2, 1:500 dilution; anti-VAMP3, 1:500 dilution, and anti-β-Tubulin (Sigma-Aldrich, clone TUB 2.1), 1:2000 dilution. The secondary antibodies used for immunodetecion were: goat anti-mouse IgG-HRP antibody (80 pg/μl, Jackson ImmunoResearch Inc) or goat anti-rabbit IgG-HRP antibody (1:10000, Cell Signaling Technology). The immunoreactive signals were visualized using ECL Advance Western Blotting System (GE Healthcare) and recorded using ImageQuant LAS-4000 (Fujifilm).
2.6. Oocyte microinjection
Microinjections were performed according to de Paola et al, 2015. MII oocytes were microinjected with anti-VAMP1, anti-VAMP2, anti-VAMP3 antibodies, using the same primary antibodies as in Immunocytochemistry and Immunoblotting sections, mouse IgG isotype control (Novus Biologicals), or rabbit IgG isotype control (Novus Biologicals). Concentration was 1 μg/μl for Anti-VAMP2, anti-VAMP3, mouse IgG isotype control (Novus Biologicals), and rabbit IgG isotype control (Novus Biologicals), the highest possible for antibodies. For VAMP1 polyclonal rabbit antiserum there is no concentration information supplied by manufacturer, in this case no dilution was performed; all antibodies and isotype controls were prepared in PBS. For tetanus toxin experiments, MII oocytes were microinjected with 10 μM tetanus toxin (TeTx) and, when indicated, were treated with 10 μM TPEN during 15 min at 37 °C prior activation with SrCl2. For microinjection, needles were filled at the indicated concentrations with injection solutions, and about 7–10 pl were injected into the cytoplasm of MII oocytes by pneumatic pressure using a Pico-Injector (model PLI-100, Harvard Apparatus, Holliston, MA). Injected oocytes were used in CGE experiments after at least 1 h incubation in M16 medium, in a humidified atmosphere with 5% CO2 at 37 °C. The number of oocytes used for each experiment is indicated in the figure legends.
2.7. Tetanus toxin (TeTx) recombinant protein purification
Plasmid pQE-3 encoding His6-tagged recombinant light chain-tetanus toxin was gently gifted by Dr. C. Tomes. Purification of His6-tagged recombinant proteins was performed under native conditions in accordance with Qiagen’s instructions, except for the fact that the 50 mM phosphate pH 8 was replaced for 50 mM TrisHCl pH 7.4 in the purification buffers. The concentration of NaCl in all buffers was 500 mM. Lysis buffer, washing buffer and elution buffer contained 20 mM, 50 mM and 350 mM of imidazole, respectively. Bradford method (Biorad) was used to determine the protein concentration. Bovine serum albumin was used as a standard for the calibration curve and the samples were quantified on a Multiskan FC (Thermo Scientific) microplate reader. For microinjection, the purified proteins were desalted by Gel filtration using Sephadex G-25 (MP Biomedicals).
2.8. SrCl2 activation of Metaphase II oocytes
Strontium chloride (SrCl2) was used for parthenogenetic activation of MII oocytes. The oocytes were thoroughly washed in calcium/magnesium-free CZB (85,35 mM NaCl, 4,83 mM KCl, 1,18 mM KH2PO4, 110 μM EDTA.2Na, 12 mM NaHCO3 25, 270 μM Na pyruvate, 52 mM Na lactate, supplemented with 0,001% Gentamicin, 0.01% PVA, 1 mM Glutamine) and then activated with freshly prepared SrCl2 (30 mM) in calcium/magnesium-free CZB for 1h at 37 °C, in a humidified atmosphere of 5% CO2 in air. Control and activated oocytes were subjected to the same incubation times. After activation, control and MII oocytes were immediately processed for CG staining.
2.9. Cortical granules staining and quantification
Staining and quantification of CG were performed according to our previous work [4;13]. Control MII oocytes and parthenogenetically activated oocytes with SrCl2. were briefly incubated in acidified Tyrode’s solution pH 2.2 to remove zona pellucida. Then, they were washed in MEM/HEPES and fixed in 3.7% PAF diluted with Dulbecco’s PBS (DPBS) for 1 h at room temperature. After fixation, cells were washed three times in BS and permeabilized with 0.1% Triton X-100 diluted with DPBS for 15 min. Afterwards, cells were washed in BS and incubated in FITC-LCA (25 μg/ml) diluted with BS for 30 min. Finally, cells were washed, mounted under partial compression between slide and coverslip in Vectashield mounting medium, sealed, and stored at 4 °C. The images on flat optical fields of cortex were acquired with a confocal laser-scanning microscope (FV1000, Olympus) using a PLAPON 60 x/NA 1.42 oil-immersion objective lens, 512 × 512 pixel resolution. The confocal acquisition parameters remained constant for all captured images within the same experiment. The mean obtained from the counting of CG present in four non overlapping equal areas from the oocyte cortex, was used to determine CG density per 100 μm2 (CG/100 μm2) for each cell, using the computer-assisted image quantification software Image J (version 1.42l; NIH, MD). For each condition, relative CG density/100 μm2 was calculated from the ratio between the mean density of CGs of the treated group to the mean density of CGs of the untreated control group, according to the following equation: [density of CGs in treated group/density of CGs in untreated group] x100, thus setting density of CGs in untreated group (control condition) as 100%.
2.10. Data analysis
All the presented experiments were repeated at least three times. The number of oocytes used for each experiment is indicated in the figure legends or below bars in graphs. Statistical significance was determined by One-Way Analysis of Variance (ANOVA) followed by Tukey’s test for multiple comparisons using KyPlot software. Data are expressed as mean ± SEM and only p < 0.05 is considered statistically significant.
3. RESULTS
3.1. Expression and localization of VAMP1, VAMP2 and VAMP3 in mouse oocytes
It has been described that some components of SNARE complex are present in mouse oocytes. Indeed, the Q-SNAREs Syntaxin4 and SNAP23 have been previously characterized [28;34]. However, it is unknown whether the R-SNAREs –or VAMPs- are involved in CGE. Of all the known VAMP isoforms that might participate as the R-SNARE in the cortical reaction, only VAMP1, VAMP2 and VAMP3 are involved exclusively in exocytosis in several secretion models [33;36;42]. So, to further characterize the molecular mechanism of CGE in mouse oocytes, we focused on these three VAMP isoforms. Considering that both germinal vesicle (GV)-intact and MII oocytes are transcriptionally inactive and that during mouse oocyte maturation (from GV to MII stage) there is significant transcript degradation [38;43;49], we extracted mRNA from GV oocytes to optimize the results. The presence of mRNA for the VAMP1, VAMP2 and VAMP3 was assessed by RT-PCR. We reverse transcribed cDNA samples from mRNA isolated from GV-intact oocytes and amplified them using the respective specific primers (Fig. 1A). The same amount of oocyte equivalents was used for all reactions. Amplified products were observed in VAMP1 (Fig. 1B, lane 2) and VAMP3 (Fig. 1B, lane 8) lanes, showing that VAMP1 and VAMP3 mRNA were expressed in GV-intact ocytes. VAMP2 showed a barely noticeable band indicating that mRNA from VAMP2 gene was scarcely transcribed in GV oocytes (Fig. 1B, lane 5). Since the gene expression of VAMP1, VAMP2 and VAMP3 has already been characterized in brain, this tissue was used as positive control. Amplification products from mouse brain cDNA were observed in all lines of VAMP (Fig. 1B, lanes 1, 4, 7). No amplified DNA fragments were observed when PCR was performed without cDNA sample (Fig. 1B, lanes 3, 7, 9) or without reverse transcriptase (Fig. 1B, lanes 10 and 11). These results showed that the VAMP1 and VAMP3 genes are the genes that are mainly expressed in mouse oocytes. Our findings are in concordance with the fact that VAMP2 is the most abundant isoform in brain and the other two isoforms, VAMP1 and VAMP3, are mostly abundant in other secretory tissues.
Figure 1. Gene expression of VAMP1, VAMP2 and VAMP3 in mouse oocyte by RT-PCR.
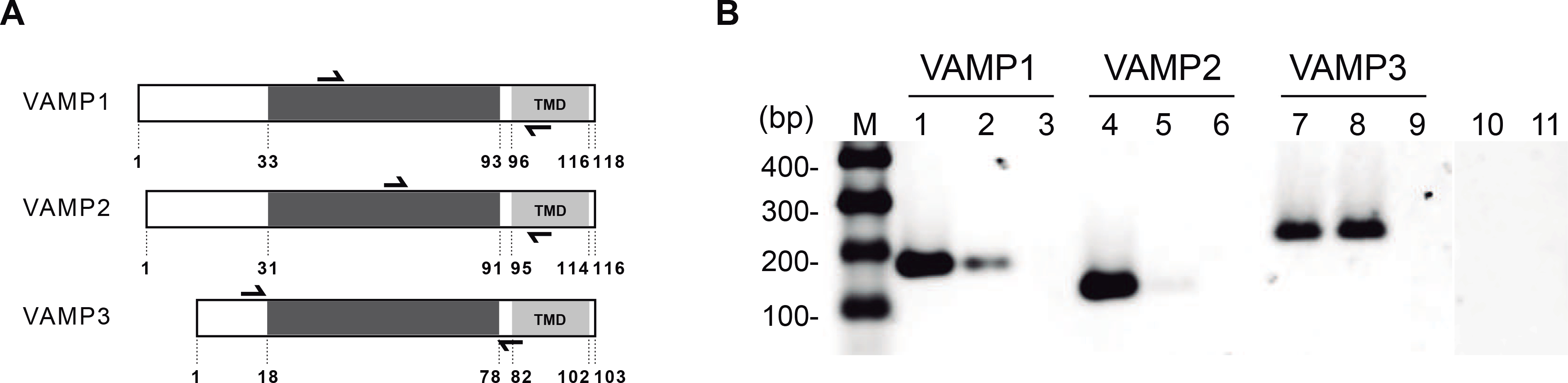
A. Domain diagrams of VAMP1, VAMP2 and VAMP3. Black arrowheads over and under each protein scheme represent forward and reverse primers for hybridation zone, respectively. Numbers under diagrams indicate the number of amino acid of VAMPs isoforms. B. RT-PCR: Lanes 1–3 and 10–11 were amplified using VAMP1 primers; lanes 4–6, using VAMP2 primers; and lanes 7–9, using VAMP3 primers. All reactions were performed under the same experimental procedure. Agarose gel was stained with SYBR Safe. Lanes: M, molecular weight marker 100–1000 bp; 1, 4, 7, mouse brain cDNA; 2, 5, 8, GV oocyte cDNA; 3, 6, 9, PCR negative controls without cDNA (non template control); 10, 11, RT-PCR negative controls without reverse transcriptase for brain and oocytes samples, respectively, using VAMP1 primers. Shown is a representative image of three independent experiments.
Then, we determined the expression of VAMP1, VAMP2 and VAMP3 proteins by Western blot in mouse oocytes. For this purpose, we collected Metaphase II (MII) oocytes, since this is the oocyte maturation stage in which cortical reaction occurs. Proteins extracted from MII oocytes and positive control tissue were resolved by SDS-PAGE, transferred to PVDF membranes and probed with the antibody of interest. We used the same antibodies used by Gadellás group to characterize VAMP1, VAMP2, and VAMP3 in porcine oocytes [51]. For VAMP1 detection, the immunoblot analysis demonstrated the presence of a protein band that comigrated with mouse brain used as positive control (Fig. 2A, left panel). Western blot analysis for VAMP2 showed that a band of the expected molecular weight was present in mouse brain, however no band was observed in mouse oocytes (Fig. 2B, center panel). Similar assays were performed for VAMP3 detection. In this case, we showed adipose tissue as a positive control since VAMP3 protein expression is more abundant in adipocytes [12]. As shown in Figure 2 (right panel), a band of the expected molecular weight was observed in the positive control and MII oocytes. These results showed that only VAMP1 and VAMP3 protein are expressed in mouse oocytes and that, in our work conditions, VAMP2 was not detected in these cells.
Figure 2. Expression and immunolocalization of VAMP1, VAMP2, and VAMP3 in mouse oocytes.
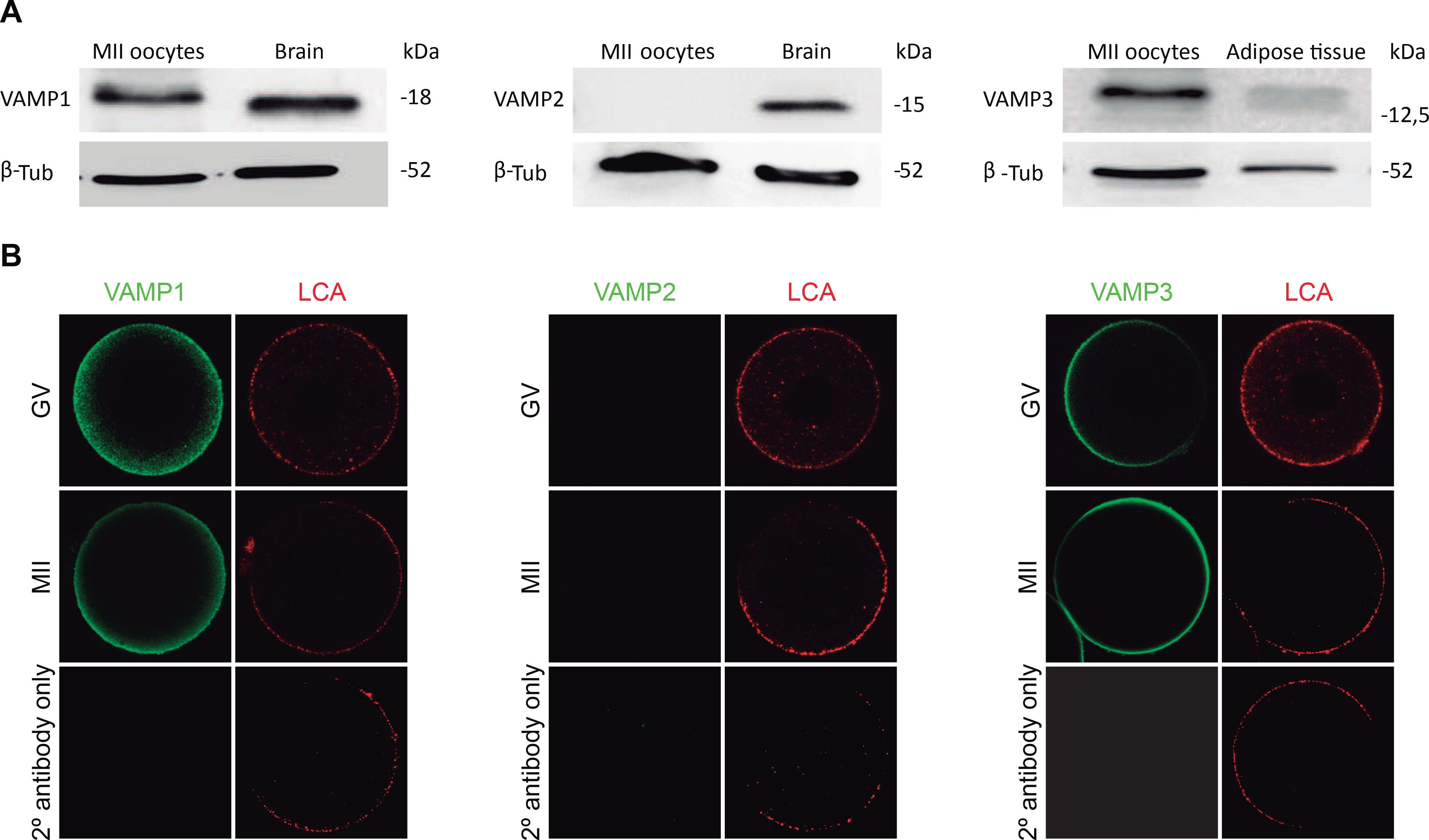
A. Immunoblot analysis of VAMP1, 2, and 3 proteins by SDS-PAGE on 15% gel. Lanes: MII oocytes: 400 MII oocytes; Brain: 1,273 μg and 0,6365 μg brain total proteins for VAMP1 and 2, respectively; Adipose tisuue: 4,74 μg mouse abdominal adipose tissue total proteins. Detection of β-tubulin was performed as a control of protein loading. Representative blots are shown (n=3). B. Immunolocalization of VAMP1, 2, and 3 proteins in GV-intact (GV) and Metaphase II (MII) oocytes. VAMP1, 2, and 3 were detected by indirect immunofluorescence. Cells were mounted in chamber under minimal compression. Confocal microscopy images of GV and MII oocytes were taken in the equatorial section of the cells. Green in each representative photomicrograph indicate positive staining for primary antibody anti-VAMP 1, 2 or 3 detected by secondary antibodies conjugated to Dye Light 488. Red represents cortical granules (CG) staining achieved with Lens Culinaris Aglutinin (LCA) conjugated to rhodamine. Images were taken at equatorial focal plane. Scale bar: 20 μm. Every experiment was performed at least three times.
Next, we analyzed the localization of VAMP1, VAMP2 and VAMP3 in GV and MII oocytes. As shown in Figure 2B, VAMP1 staining was mainly concentrated in the cortex region at both stages (Fig. 2B, left panel). In MII oocytes, the fluorescencés intensity of VAMP1 was stronger in the cortical region. When the immunolocalization of VAMP2 was assayed no signal was observed at any maturation stage. In fact, the central panel of Figure 2B showed no staining for VAMP2 in GV and MII oocytes. Similarly to VAMP1, VAMP3 showed a sharp localization in the cortical region of GV and MII oocytes (Fig. 2, right panel). For all immunofluorescence experiments, no signal was observed when the specific primary antibody was omitted (Fig. 2B, see 2° antibody only panel). Altogether, these results showed that VAMP1 and VAMP3, but not VAMP2, isoforms are expressed and localized in the cortical region of MII oocytes, which is enriched with cortical granules (see LCA staining in Fig. 2B). This cortical localization prompted us to investigate the involvement of these proteins in CGE.
3.2. Cortical granule exocytosis is sensitive to tetanus toxin
Cortical granule exocytosis is a calcium regulated exocytosis, in which CG fuse with the oocytés plasma membrane after mouse oocyte activation. This fusion is thought to be mediated by SNAREs. The SNARE hypothesis of membrane fusion was first supported by the demonstration that the neuronal synaptic v-SNARE, VAMP2, and t-SNAREs, Syntaxin1 and SNAP25, are proteolyzed by the light chains of the clostridial neurotoxins – tetanus toxin and botulinum toxin [26;41]. These toxins have two polypeptide chains: the heavy and the light chain. The heavy chain mediates binding, internalization, and translocation of the light chain to the cytosol, and the light chain inhibits synaptic transmission by cleaving either VAMP2, Syntaxin, or SNAP25 at specific and single sites [16]. The catalytic activity of the light chain is zinc-dependent and is used as a tool for the study of exocytosis in different secretory cells [1;7;25]. VAMP1, VAMP2 and VAMP3 are the only known R-SNAREs to be sensitive to the light chain of tetanus toxin (TeTx) [26]. However, this toxin is not active when SNARE complex is preassembled [23;39]. Using a functional assay, we previously demonstrated that alpha-SNAP/NSF complex participates in CGE [13]. This finding indicates that, unlike other secretory processes, SNARE proteins are preassembled in mouse oocytes and need to be disassembled by alpha-SNAP/NSF complex to allow the fusion of CG with the oolema [13]. Therefore, if tetanus toxin were present during the disassembling of SNARE complexes occurring in activated oocytes, the identified VAMP isoforms –VAMP1 and VAMP3– might be available for toxin activity. Hence, the VAMP isoforms would be cleaved and CGE would be impaired. We tested this hypothesis by microinjecting tetanus toxin in oocytes prior strontium activation. Zona pellucida of the treated oocytes was removed before fixation and CG were stained with FITC-Lens Culinaris Agglutinin (LCA) to evaluate CG density as a measure of cortical reaction (see Materials and Methods section for details). As shown in figure 3, the microinjection of tetanus toxin significantly inhibited the cortical reaction activated by SrCl2 (Fig. 3) indicating that VAMP1 or VAMP3 might be involved in CGE. Tetanus toxin inhibited significantly cortical reaction by about 50 %, suggesting that other tetanus toxin-insensitive protein might be involved in this secretory process.
Figure 3. Effect of tetanus toxin microinjection on cortical granule exocytosis.
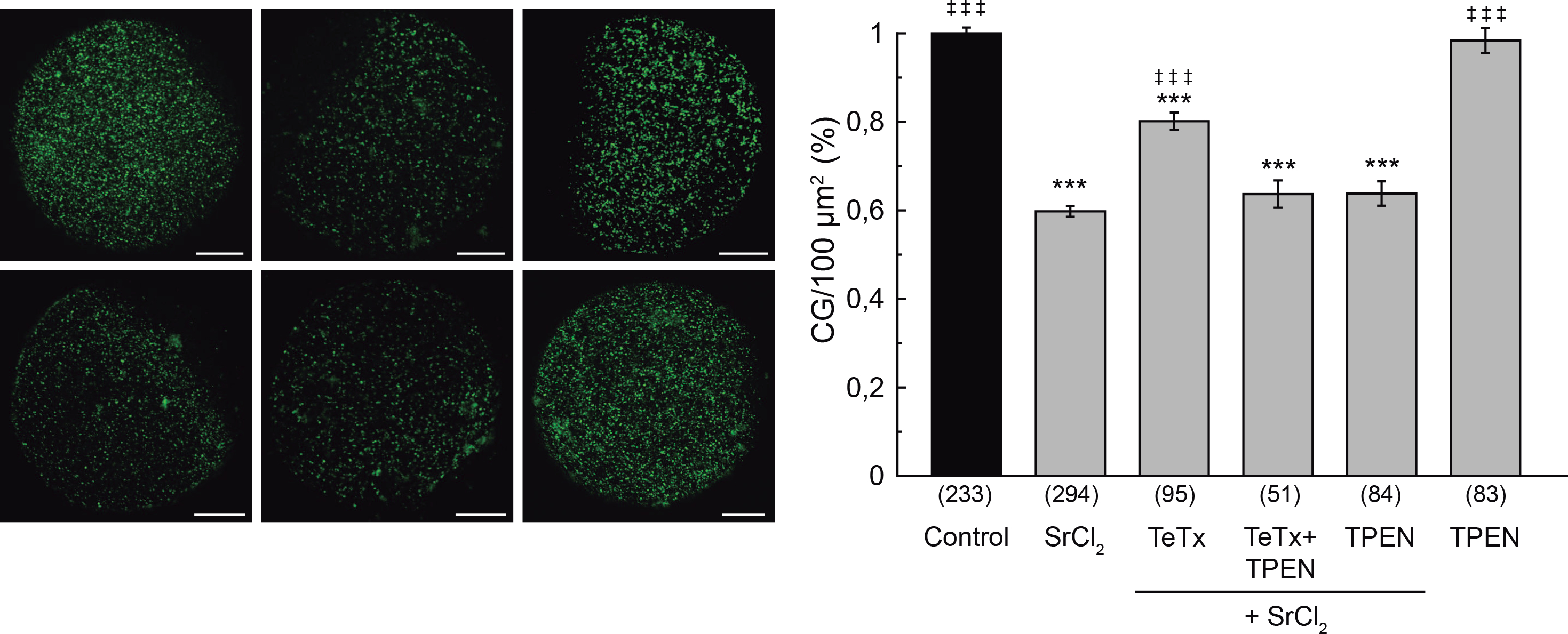
Oocytes were microinjected with 10 μM tetanus toxin (TeTx), and when indicated, incubated with TPEN 10 μM. Cortical granule exocytosis was triggered with 30 mM SrCl2. Images were taken as described in M&M. Left, representative confocal images of oocytes stained with FITC-LCA for each experimental condition. Scale bar: 20 μm. Right, histogram showing CG density/100 μm2 for different treatments, relative to untreated group (Control) set as 100%. Data are shown as mean ± SEM from 3 independent experiments. Numbers in parentheses below bars represent total number of oocytes. ***, values compared to control without stimulus, p ≤ 0,001; ‡ ‡ ‡, values compared to SrCl2, p ≤ 0,001. Statistical tests: One way ANOVA and Tukey’s test.
Next, considering that the catalytic activity of the light chain of tetanus toxin is zinc-dependent, we analyzed the effect of zinc chelation to demonstrate the specificity of this toxin effect. We used N,N,N′,N′-Tetrakis(2-pyridylmethyl) ethylenediamine (TPEN), a cell-permeable zinc chelator with a high affinity for zinc. Incubation of tetanus toxin-microinjected oocytes in 10 μM TPEN prevented the inhibition of CGE, confirming the specificity of the effect of tetanus toxin (Fig. 3). TPEN incubation alone did not alter the cortical reaction in presence or absence of SrCl2 (Fig. 3). These results confirmed our hypothesis and demonstrated that CGE is sensitive to tetanus toxin.
3.3. VAMP1 and VAMP3 have an active role in cortical granule exocytosis
In previous sections we have demonstrated that VAMP1 and VAMP3 are expressed in mouse oocytes and that the tetanus toxin inhibited cortical reaction. These results suggested that VAMP1 or VAMP3 might be involved in the cortical reaction. A very useful technique to demonstrate the participation of a protein in cortical reaction is blocking the function of endogenous protein by antibody microinjection prior to oocyte activation [4;13]. As we have shown previously, CGE assay is a functional assay that measures the secretory process of cortical granules and, indirectly, can measure the antibody blocking function by virtue of specific steric interference. If CGE is activated in oocytes previously microinjected with a blocking antibody, CGE will be inhibited because the antibody will block the function of the endogenous protein. On the contrary, if the microinjected antibody does not block CGE, it means that no protein was recognized by the antibody microinjected. Even more, to demonstrate that the antibody blocking effect is specific, an IgG isyotype control antibody is microinjected and it is expected to have no effect. Hence, to confirm the participation of VAMP1 and VAMP3, but not VAMP2, in the cortical reaction, we perturbed the endogenous protein by microinjecting mouse MII oocytes with anti-VAMP1, anti-VAMP2, or anti-VAMP3 antibody prior strontium activation. After cell fixation and CG staining, the CG density was evaluated as described in the previous section (Fig. 4). The microinjection of anti-VAMP1 antibody significantly inhibited the CGE (Fig. 4A). This result can be easily observed if control and VAMP1 Ab images are compared; note that CG density is similar in both images, which indicates that CG were not secreted when anti-VAMP1 antibody was microinjected. The microinjection of a rabbit IgG isotype control had no effect, showing that microinjection procedure or an unspecific IgG were not responsible for the observed inhibition (Fig. 4A). In this case, note that the CG density in rabbit IgG image is similar to activated oocytes (positive control, SrCl2-activated oocytes), indicating that CG were secreted in oocytes microinjected with rabbit IgG. In contrast, the microinjection of anti-VAMP2 antibody in MII oocytes was not able to inhibit the cortical reaction activated by strontium chloride (Fig. 4B), indicating that this isoform does not have a role in this secretory process. Here, note that VAMP2 Ab image is similar to both activated (SrCl2) and isotype IgG (Mouse IgG)-microinjected oocytes, indicating that CGs were secreted. Finally, the microinjection of the anti-VAMP3 antibody specifically inhibited the cortical reaction stimulated by SrCl2 (figure 4C), while the rabbit IgG isotype control had no effect on the cortical reaction. In this case, as VAMP1 Ab image, VAMP3 Ab image shows similar CG density when compared with control image, indicating that the CGs were not secreted when anti-VAMP3 antibody was microinjected. Altogether, these results demonstrate that VAMP1 and VAMP3, but not VAMP2, have an active role and are required for CGE in mouse oocytes.
Figure 4. Effect of microinjection of anti-VAMP1, anti-VAMP2 and anti-VAMP3 antibodies on cortical granule exocytosis.
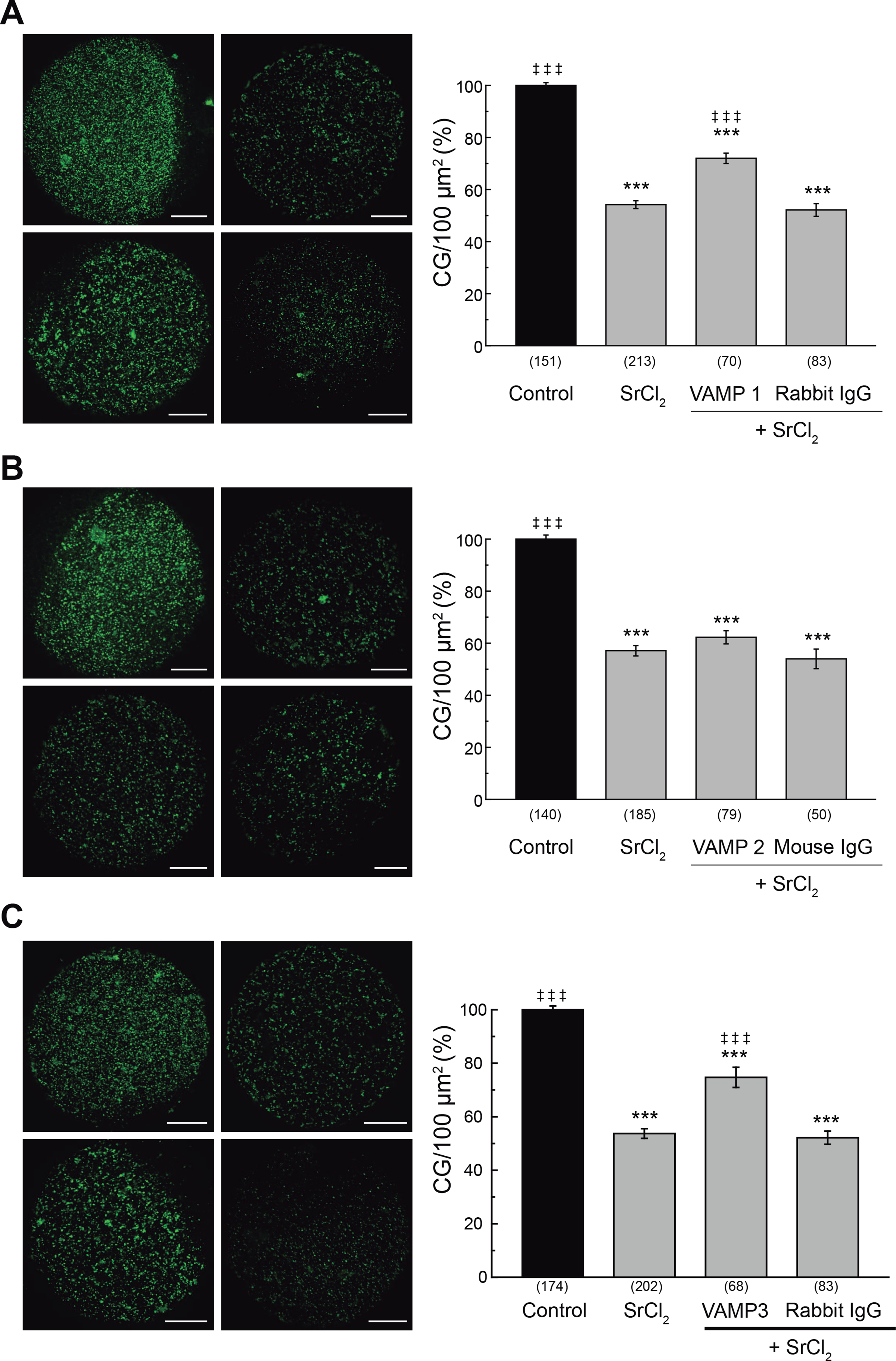
Oocytes were microinjected with either anti-VAMP1 (A), anti-VAMP2 (B), or anti-VAMP3 (C) antibodies (1 μg/μl) and the cortical reaction was triggered with 30 mM SrCl2. Rabbit or mouse IgG were microinjected as isotype controls. Images were taken as described in M&M. Left, for each panel: representative confocal microscopic images of oocytes stained with FITC-LCA. Scale bar: 20 μm. Right, for each panel: histogram showing CG density/100 μm2 for different treatments and relative to untreated group (Control) set as 100%. Data are shown as mean ± SEM from at least 3 independent experiments. Numbers in parentheses below bars represent total number of oocytes. ***, values compared to control (without stimulus), p ≤ 0,001; ‡ ‡ ‡, values compared to SrCl2, p ≤ 0,001. Statistical tests: One way ANOVA and Tukey’s test.
4. DISCUSSION
In this work, we aim to investigate if R-SNAREs involved exclusively in secretory processes –VAMP1, VAMP2, or VAMP3 – participate in CGE. We demonstrated by RT-PCR that VAMP1 and VAMP3 were the isoforms expressed in mouse oocytes (Fig. 1). Immunoblot also demonstrated that VAMP1 and VAMP3, but not VAMP2, were detected in these cells (Fig. 2A). The immunolocalization showed that VAMP1 and VAMP3 were observed in the cortical region of GV and MII mouse oocytes, whereas VAMP2 was not observed at any of these maturation stages (Fig. 2B). It is worth pointing out that even using the same VAMPs antibodies than Gadellás group, our results are different from those obtained in porcine oocytes, in which the absent isoform was VAMP3 [51]. This suggests that the molecular components of CGE may be similar but not identical among mammalians. Knowing that VAMP1, VAMP2, and VAMP3 are the only known R-SNAREs to be sensitive to tetanus toxin [26], we assayed the effect of tetanus toxin during oocyte activation using the functional assay described by our group [4;13]. Previously, we demonstrated that alpha-SNAP/NSF complex participates in CGE [13] indicating that SNARE proteins are preassembled in mouse oocytes and need to be disassembled by alpha-SNAP/NSF complex to allow the fusion of CG with the oolema [13]. According to our prediction, CGE was impaired when tetanus toxin was present during the mouse oocyte activation (Fig. 3), indicating that at least one toxin-sensitive VAMP was necessary for CGE. To demonstrate that VAMP1 or VAMP3 were active during cortical reaction, we perturbed the endogenous protein by the specific antibody microinjection. Only the microinjection of anti-VAMP1 or anti-VAMP3 was able to inhibit CGE (Fig. 4), demonstrating that VAMP1 and VAMP3 participate in CG fusion with the oolema.
Unlike the work of Tsai et al that described the presence of VAMP1 and VAMP2 –but not VAMP3– by Western blot and indirect immunofluorescence in porcine oocytes [51], here, we show that VAMP1 and VAMP3 –but not VAMP2– are expressed and localized in the cortical region of mouse oocytes. Furthermore, we demonstrate the involvement of these VAMPs sensitive to tetanus toxin in cortical reaction by a functional assay in live cells. In other words, our work not only reports the expression and localization of VAMP1 and VAMP3 but also shows that blocking of the endogenous proteins by the corresponding antibody microinjection inhibited CGE in mouse activated oocytes. Nevertheless, considering that 9 VAMP isoforms have been predicted in humans [8] and that only 7 VAMP isoforms have been characterized in rodent models, we cannot exclude that other VAMP isoforms insensitive to tetanus toxin may be involved in CGE. In fact, the microinjection of tetanus toxin inhibited significantly cortical reaction by about 50 %, suggesting that other tetanus toxin-insensitive protein might be involved in this secretory process.
SNAREs proteins are the engine of membrane fusion during regulated exocytosis [29]; however, other conserved sets of protein families that include GTPases of the Rab family, the Sec1/Munc-18 protein family, and Synaptotagmin family are necessary for this process. Rab GTPases are essential regulators of membrane trafficking [48] and can recruit other Rabs or proteins such as Rabphilin [19;40]. Rab3A and Rab27A can cooperatively regulate the tethering and docking step of vesicles to the plasma membrane in PC12 cells [52], in endocrine cells [15] and human sperm [5;11;55]. In mouse oocytes, Rab3A, Rab27A, and Rabphilin3A have been identified and involved in CGE [4;32;54]. Rabs 3A and 27A colocalize with cortical granules [54], and microinjection of function-blocking antibodies [4] or protein depletion using RNAi [54] inhibits CGE [4;54]. Moreover, Rabphilin 3A, an effector of Rab3A, has been involved in cortical reaction since the microinjection of the NH2- or COOH-terminal fragment of recombinant Rabphilin-3A into mouse MII oocytes inhibited CGE in a dose-dependent manner [15].
Another highly conserved family of proteins is Synaptotagmin [18]. Synaptotagmin1 is a calcium sensor, abundant in the synaptic vesicle membrane and plays an essential role in neurotransmitter release. Zanetti et al showed that the C2AB portion of Synaptotagmin1 could self-assemble into calcium-sensitive ring-like oligomers on membranes to regulate neurotransmitter release [56]. Recently, Zhu et al have shown that Synaptotagmin1 knockdown by Synaptotagmin1 specific-domain morpholino was found to affect intracellular [Ca2+] oscillations, the F-actin organization, and CGE in mouse oocytes, demonstrating that Synaptotagmin1 regulates CGE [57]. So far, no studies have been conducted to investigate if the Sec1/Munc-18 protein family is expressed in mouse oocytes.
Cortical granules are membrane-bound organelles that are derived from Golgi apparatus and appear in the early stages of oocyte growth. In fact, in mouse oocytes, these granules are first observed in the unilaminar follicles [31]. During follicular growth, small vesicles are formed from hypertrophied Golgi complexes and, then, coalesce to form mature CG [22]. Hence, CG formation and maturation involved previous membrane fusion steps in which ternary cis-SNARE complexes can be acquired for CG membrane. There is no evidence about whether SNAREs are forming cis-SNARE complexes in mammalian oocytes; however, findings from this and our previous work suggest that SNAREs are preassembled in CG of mouse oocytes [13].
Regarding the Q-SNAREs involved in CGE, only SNAP23 has been identified as a SNARE component in the membrane fusion of cortical granules with plasma membrane in mouse oocytes [34]. During two decades it was believed that SNAP25 was present in mouse oocytes and that it was one of the Q-SNARE required for regulated exocytosis in mouse eggs [27]. However, a recent work from Mehlman et al. demonstrated that SNAP23, but not SNAP25, is present in mouse oocytes and that it is required for regulated exocytosis, exerting its function downstream of Ca2+ release [34]. On the other hand, Syntaxin4 was identified almost twenty years ago in mouse eggs [28], but its function in CGE has not been demonstrated.
In order to summarize our results and those of other groups presented throughout this discussion, we present a working model for membrane fusion during cortical reaction in mouse oocytes (Fig. 5). We proposed that ternary cis-SNAREs complexes are preformed on CG (and probably on plasma membrane remnant from constitutive exocytosis [34]). During mouse oocyte activation, a sudden increase in the amount of calcium ions would activate alpha-SNAP/NSF complex, which would release cis-SNARE components to allow the fusion of CG and plasma membrane [13]. Then, Rab3A, Rab27A and Rabphilin, which are reported to participate in CGE regulation [4;32;54], would facilitate the CG tethering and docking to the plasma membrane, probably acting as an anchor to the plasma membrane to control the fusion of cortical granules. Finally, Synaptotagmin1 [57] would detect calcium ions and would bind to the cell membrane and the new trans-SNAREs complexes allowing the secretion of CG content (Fig.5).
Figure 5. Working model for membrane fusion during cortical granule exocytosis in mouse oocytes.
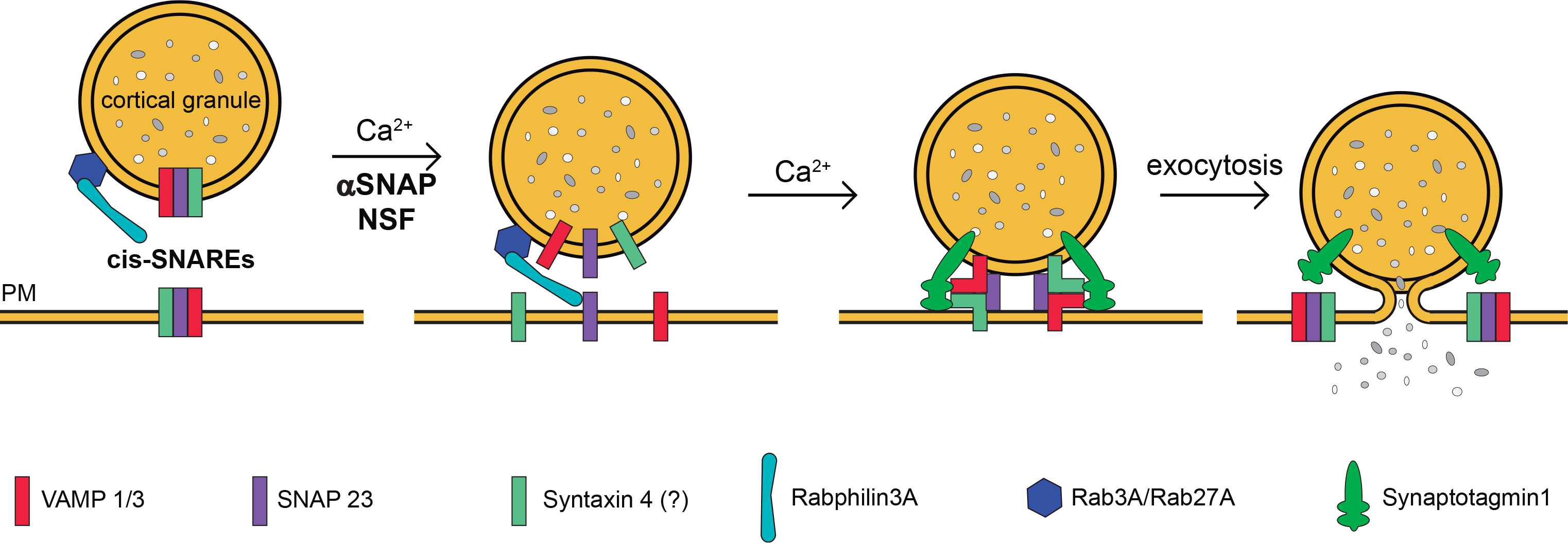
Based on our previous and recent results and those published by other groups, we present this scheme to summarize the proteins that are involved in cortical granules exocytosis in mouse oocytes and their probable functions. We proposed that ternary cis-SNAREs complexes –formed by SNAP23 [34], Syntaxin4 [28] and VAMP1/3 (this work) are preformed on cortical granules (and probably on plasma membrane remnant from constitutive exocytosis). Rab3A-GTP [4], Rabphilin3A [32], and Rab27-GTP [54] are already recruited on cortical granules. During mouse oocyte activation, a sudden increase in the amount of calcium ions (Ca2+) would activate alpha-SNAP/NSF complex and Synaptotagmin1. Alpha-SNAP/NSF complex would disassemble preformed SNARE complexes [13] and would release their components to allow the fusion of vesicle granules and plasma membrane (PM). Meanwhile, Rabs/rabphilin complex would facilitate the tethering membranes. Finally, Synaptotagmin1[57] would detect calcium ions and would bind to the cell membrane and the new trans-SNAREs complexes allowing the secretion of cortical granule content.
One raised question from our work is: why are more than one VAMP isoforms involved in the cortical reaction? Interestingly, SNAREs can functionally replace each other to a certain extent. For instance, VAMP2 and VAMP3 are capable of substituting for each other to a varying degree in the regulated exocytosis of chromaffin cells [9]. Similarly, SNAP23 can rescue exocytosis in embryonic chromaffin cells from SNAP25 null mice [47]. Probably the coexistence of more than one isoform allows ensuring an efficient cortical reaction that avoids polyspermy, securing the development of the embryo. In fact, if VAMP3 were indispensable for CGE we might predict that VAMP3 knock-out mice would be infertile. However, VAMP3 knock-out mice are viable and fertile [9]. According to our findings, we speculate that these mice are fertile because VAMP1 is enough to support the cortical reaction. Nevertheless, this should be further explored.
Based on published studies which suggest that SNAREs are also important in the control of tumorigenesis through the regulation of multiple signaling and transportation pathways [35], we speculate that SNARE and associated proteins would also be a target for contraception. In fact, we have recently characterized the oocyte phenotype from hyh mice that have a point mutation in the alpha-SNAP gene and have demonstrated that alterations in SNARE-related proteins affect female fertility [2;14].
In conclusion, our results reveal that CGE is sensitive to tetanus toxin and two out of the three tetanus toxin sensitive VAMPs –VAMP1 and VAMP3– are required for CGE. This work completes the characterization of the SNARE proteins in the cortical reaction and finally demonstrates that the fusion of CG with the oolema is mediated by the SNARE complex.
HIGHLIGHTS.
Cortical granule exocytosis is sensitive to tetanus toxin in mouse oocytes
VAMP1 and VAMP3 proteins participate in cortical granule exocytosis
This work confirms that cortical granule exocytosis is mediated by SNARE complex
ACKNOWLEDGMENTS
The authors are very grateful to Dr. Mariano Polo, for his critical reading of the manuscript, and Dr. Claudia Tomes and Dr. Luis Mayorga for their contribution to the working model. The authors also thank Dr. Claudia Tomes for the plasmid pQE-3 encoding His6-tagged recombinant light chain tetanus toxin and acknowledge the help of Lucas Aldao and Veterinarian Julieta Scelta in the maintenance of the animal facility. M. dP. and N.Z. are thankful to CONICET, Argentina, for fellowships. F.G. is thankful to Consejo Interuniversitario Nacional and Universidad Nacional de Cuyo, Argentina, for fellowship.
FUNDING
This work was funded by Fogarty International Center from NIH, USA (GRIP, R01 TW007571), Agencia Nacional de Promoción Científica y Tecnológica (PICT 2012-0218), and Universidad Nacional de Cuyo (06/M071 and 06/M093), Argentina, to MAM.
Abreviations
- CG
cortical granules
- CGE
cortical granule exocytosis
- VAMP
vesicle associated membrane protein
- MII oocytes
Metaphase II oocytes
- GV
germinal vesicle
- LCA
lens culinaris agglutinin
Footnotes
DECLARATION OF INTEREST
None
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- [1].Ahnert-Hilger G, Weller U, Dauzenroth ME, Habermann E, Gratzl M, The tetanus toxin light chain inhibits exocytosis. FEBS Lett. 242 (1989) 245–248. [DOI] [PubMed] [Google Scholar]
- [2].Arcos A, Paola M, Gianetti D, Acuna D, Velasquez ZD, Miro MP, Toro G, Hinrichsen B, Munoz RI, Lin Y, Mardones GA, Ehrenfeld P, Rivera FJ, Michaut MA, Batiz LF, alpha-SNAP is expressed in mouse ovarian granulosa cells and plays a key role in folliculogenesis and female fertility. Sci.Rep. 7 (2017) 11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Austin CR, Cortical granules in hamster eggs. Exp.Cell Res. 10 (1956) 533–540. [DOI] [PubMed] [Google Scholar]
- [4].Bello OD, Cappa AI, de PM, Zanetti MN, Fukuda M, Fissore RA, Mayorga LS, Michaut MA, Rab3A, a possible marker of cortical granules, participates in cortical granule exocytosis in mouse eggs. Exp.Cell Res. 347 (2016) 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bello OD, Zanetti MN, Mayorga LS, Michaut MA, RIM, Munc13, and Rab3A interplay in acrosomal exocytosis. Exp.Cell Res. 318 (2012) 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bennett MK, Calakos N, Scheller RH, Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science 257 (1992) 255–259. [DOI] [PubMed] [Google Scholar]
- [7].Bittner MA, Habig WH, Holz RW, Isolated light chain of tetanus toxin inhibits exocytosis: studies in digitonin-permeabilized cells. J.Neurochem. 53 (1989) 966–968. [DOI] [PubMed] [Google Scholar]
- [8].Bock JB, Matern HT, Peden AA, Scheller RH, A genomic perspective on membrane compartment organization. Nature 409 (2001) 839–841. [DOI] [PubMed] [Google Scholar]
- [9].Borisovska M, Zhao Y, Tsytsyura Y, Glyvuk N, Takamori S, Matti U, Rettig J, Sudhof T, Bruns D, v-SNAREs control exocytosis of vesicles from priming to fusion. EMBO J. 24 (2005) 2114–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Burkart AD, Xiong B, Baibakov B, Jimenez-Movilla M, Dean J, Ovastacin, a cortical granule protease, cleaves ZP2 in the zona pellucida to prevent polyspermy. J.Cell Biol. 197 (2012) 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bustos MA, Lucchesi O, Ruete MC, Mayorga LS, Tomes CN, Rab27 and Rab3 sequentially regulate human sperm dense-core granule exocytosis. Proc.Natl.Acad.Sci.U.S.A 109 (2012) E2057–E2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cain CC, Trimble WS, Lienhard GE, Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J.Biol.Chem. 267 (1992) 11681–11684. [PubMed] [Google Scholar]
- [13].de Paola M, Bello OD, Michaut MA, Cortical Granule Exocytosis Is Mediated by Alpha-SNAP and N-Ethilmaleimide Sensitive Factor in Mouse Oocytes. PLoS.One. 10 (2015) e0135679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].de Paola M, Miro MP, Ratto M, Batiz LF, Michaut MA, Pleiotropic effects of alpha-SNAP M105I mutation on oocyte biology: ultrastructural and cellular changes that adversely affect female fertility in mice. Sci.Rep. 9 (2019) 17374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Desnos C, Schonn JS, Huet S, Tran VS, El-Amraoui A, Raposo G, Fanget I, Chapuis C, Menasche G, de Saint BG, Petit C, Cribier S, Henry JP, Darchen F, Rab27A and its effector MyRIP link secretory granules to F-actin and control their motion towards release sites. J.Cell Biol. 163 (2003) 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dong M, Masuyer G, Stenmark P, Botulinum and Tetanus Neurotoxins. Annu.Rev.Biochem. 88 (2019) 811–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fasshauer D, Sutton RB, Brunger AT, Jahn R, Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc.Natl.Acad.Sci.U.S.A 95 (1998) 15781–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fukuda M The Role of synaptotagmin and synaptotagmin-like protein (Slp) in regulated exocytosis, In: Regazzi Romano, editor. Molecular mechanisms of exocytosis. Austin: Landes Bioscience; 2006; pp. 42–61, 2006. [Google Scholar]
- [19].Fukuda M, Distinct Rab binding specificity of Rim1, Rim2, rabphilin, and Noc2. Identification of a critical determinant of Rab3A/Rab27A recognition by Rim2. J.Biol.Chem. 278 (2003) 15373–15380. [DOI] [PubMed] [Google Scholar]
- [20].Gardner AJ, Evans JP, Mammalian membrane block to polyspermy: new insights into how mammalian eggs prevent fertilisation by multiple sperm. Reprod Fertil.Dev. 18 (2006) 53–61. [DOI] [PubMed] [Google Scholar]
- [21].Gomez-Elias MD, Fissore RA, Cuasnicu PS, Cohen DJ, Compensatory endocytosis occurs after cortical granule exocytosis in mouse eggs. J.Cell Physiol 235 (2020) 4351–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gulyas BJ, Cortical granules of mammalian eggs. Int.Rev.Cytol. 63 (1980) 357–392. [DOI] [PubMed] [Google Scholar]
- [23].Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Sudhof TC, Niemann H, Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 13 (1994) 5051–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hess DT, Slater TM, Wilson MC, Skene JH, The 25 kDa synaptosomal-associated protein SNAP-25 is the major methionine-rich polypeptide in rapid axonal transport and a major substrate for palmitoylation in adult CNS. J.Neurosci. 12 (1992) 4634–4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hohne-Zell B, Ecker A, Weller U, Gratzl M, Synaptobrevin cleavage by the tetanus toxin light chain is linked to the inhibition of exocytosis in chromaffin cells. FEBS Lett. 355 (1994) 131–134. [DOI] [PubMed] [Google Scholar]
- [26].Humeau Y, Doussau F, Grant NJ, Poulain B, How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie 82 (2000) 427–446. [DOI] [PubMed] [Google Scholar]
- [27].Ikebuchi Y, Masumoto N, Matsuoka T, Yokoi T, Tahara M, Tasaka K, Miyake A, Murata Y, SNAP-25 is essential for cortical granule exocytosis in mouse eggs. Am.J.Physiol 274 (1998) C1496–C1500. [DOI] [PubMed] [Google Scholar]
- [28].Iwahashi K, Kuji N, Fujiwara T, Tanaka H, Takahashi J, Inagaki N, Komatsu S, Yamamoto A, Yoshimura Y, Akagawa K, Expression of the exocytotic protein syntaxin in mouse oocytes. Reproduction. 126 (2003) 73–81. [DOI] [PubMed] [Google Scholar]
- [29].Jahn R, Scheller RH, SNAREs--engines for membrane fusion. Nat.Rev.Mol Cell Biol. 7 (2006) 631–643. [DOI] [PubMed] [Google Scholar]
- [30].Kutay U, Hartmann E, Rapoport TA, A class of membrane proteins with a C-terminal anchor. Trends Cell Biol. 3 (1993) 72–75. [DOI] [PubMed] [Google Scholar]
- [31].Liu M, The biology and dynamics of mammalian cortical granules. Reprod Biol.Endocrinol. 9 (2011) 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Masumoto N, Sasaki T, Tahara M, Mammoto A, Ikebuchi Y, Tasaka K, Tokunaga M, Takai Y, Miyake A, Involvement of Rabphilin-3A in cortical granule exocytosis in mouse eggs. J.Cell Biol. 135 (1996) 1741–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].McMahon HT, Ushkaryov YA, Edelmann L, Link E, Binz T, Niemann H, Jahn R, Sudhof TC, Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature 364 (1993) 346–349. [DOI] [PubMed] [Google Scholar]
- [34].Mehlmann LM, Uliasz TF, Lowther KM, SNAP23 is required for constitutive and regulated exocytosis in mouse oocytesdagger. Biol.Reprod 101 (2019) 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Meng J, Wang J, Role of SNARE proteins in tumourigenesis and their potential as targets for novel anti-cancer therapeutics. Biochim.Biophys.Acta 1856 (2015) 1–12. [DOI] [PubMed] [Google Scholar]
- [36].Meng J, Wang J, Lawrence G, Dolly JO, Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J.Cell Sci. 120 (2007) 2864–2874. [DOI] [PubMed] [Google Scholar]
- [37].Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC, The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J.Cell Biol. 109 (1989) 3039–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Paynton BV, Rempel R, Bachvarova R, Changes in state of adenylation and time course of degradation of maternal mRNAs during oocyte maturation and early embryonic development in the mouse. Dev.Biol. 129 (1988) 304–314. [DOI] [PubMed] [Google Scholar]
- [39].Pellizzari R, Rossetto O, Schiavo G, Montecucco C, Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Philos.Trans.R.Soc.Lond B Biol.Sci. 354 (1999) 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Quevedo MF, Bustos MA, Masone D, Roggero CM, Bustos DM, Tomes CN, Grab recruitment by Rab27A-Rabphilin3a triggers Rab3A activation in human sperm exocytosis. Biochim.Biophys.Acta Mol Cell Res. 1866 (2019) 612–622. [DOI] [PubMed] [Google Scholar]
- [41].Schiavo G, Matteoli M, Montecucco C, Neurotoxins affecting neuroexocytosis. Physiol Rev. 80 (2000) 717–766. [DOI] [PubMed] [Google Scholar]
- [42].Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET, SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science 294 (2001) 1117–1122. [DOI] [PubMed] [Google Scholar]
- [43].Sha QQ, Yu JL, Guo JX, Dai XX, Jiang JC, Zhang YL, Yu C, Ji SY, Jiang Y, Zhang SY, Shen L, Ou XH, Fan HY, CNOT6L couples the selective degradation of maternal transcripts to meiotic cell cycle progression in mouse oocyte. EMBO J. 37 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Smith RM, Baibakov B, Ikebuchi Y, White BH, Lambert NA, Kaczmarek LK, Vogel SS, Exocytotic insertion of calcium channels constrains compensatory endocytosis to sites of exocytosis. J.Cell Biol. 148 (2000) 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE, A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75 (1993) 409–418. [DOI] [PubMed] [Google Scholar]
- [46].Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE, SNAP receptors implicated in vesicle targeting and fusion. Nature 362 (1993) 318–324. [DOI] [PubMed] [Google Scholar]
- [47].Sorensen JB, Nagy G, Varoqueaux F, Nehring RB, Brose N, Wilson MC, Neher E, Differential control of the releasable vesicle pools by SNAP-25 splice variants and SNAP-23. Cell 114 (2003) 75–86. [DOI] [PubMed] [Google Scholar]
- [48].Stenmark H, Rab GTPases as coordinators of vesicle traffic. Nat.Rev.Mol Cell Biol. 10 (2009) 513–525. [DOI] [PubMed] [Google Scholar]
- [49].Su YQ, Sugiura K, Woo Y, Wigglesworth K, Kamdar S, Affourtit J, Eppig JJ, Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev.Biol. 302 (2007) 104–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Trimble WS, Cowan DM, Scheller RH, VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc.Natl.Acad.Sci.U.S.A 85 (1988) 4538–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Tsai PS, van HT, Gadella BM, Preparation of the cortical reaction: maturation-dependent migration of SNARE proteins, clathrin, and complexin to the porcine oocyte’s surface blocks membrane traffic until fertilization. Biol.Reprod 84 (2011) 327–335. [DOI] [PubMed] [Google Scholar]
- [52].Tsuboi T, Fukuda M, Rab3A and Rab27A cooperatively regulate the docking step of dense-core vesicle exocytosis in PC12 cells. J.Cell Sci. 119 (2006) 2196–2203. [DOI] [PubMed] [Google Scholar]
- [53].Veit M, Sollner TH, Rothman JE, Multiple palmitoylation of synaptotagmin and the t-SNARE SNAP-25. FEBS Lett. 385 (1996) 119–123. [DOI] [PubMed] [Google Scholar]
- [54].Wang HH, Cui Q, Zhang T, Wang ZB, Ouyang YC, Shen W, Ma JY, Schatten H, Sun QY, Rab3A, Rab27A, and Rab35 regulate different events during mouse oocyte meiotic maturation and activation. Histochem.Cell Biol. 145 (2016) 647–657. [DOI] [PubMed] [Google Scholar]
- [55].Yunes R, Tomes C, Michaut M, De BG, Rodriguez F, Regazzi R, Mayorga LS, Rab3A and calmodulin regulate acrosomal exocytosis by mechanisms that do not require a direct interaction. FEBS Lett. 525 (2002) 126–130. [DOI] [PubMed] [Google Scholar]
- [56].Zanetti MN, Bello OD, Wang J, Coleman J, Cai Y, Sindelar CV, Rothman JE, Krishnakumar SS, Ring-like oligomers of Synaptotagmins and related C2 domain proteins. Elife. 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zhu XL, Li SF, Zhang XQ, Xu H, Luo YQ, Yi YH, Lv LJ, Zhang CH, Wang ZB, Ouyang YC, Hou Y, Schatten H, Liu FH, Synaptotagmin 1 regulates cortical granule exocytosis during mouse oocyte activation. Zygote.2019) 1–6. [DOI] [PubMed] [Google Scholar]


