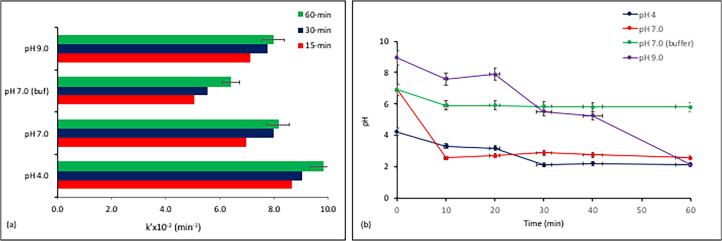
Fig. 1.
The drop in pH during sonolysis of caffeine (a), and the variations in the the apparent reaction rate constant within 15 and 30-min intervals by the initial solution pH (b). The buffering agent was sodium phosphate (0.1 M; pH 7.0), pH adjustment was made with NaOH (0.1 M), the initial values of pH and concentration were 4.0 and 5 mg L−1, respectively.