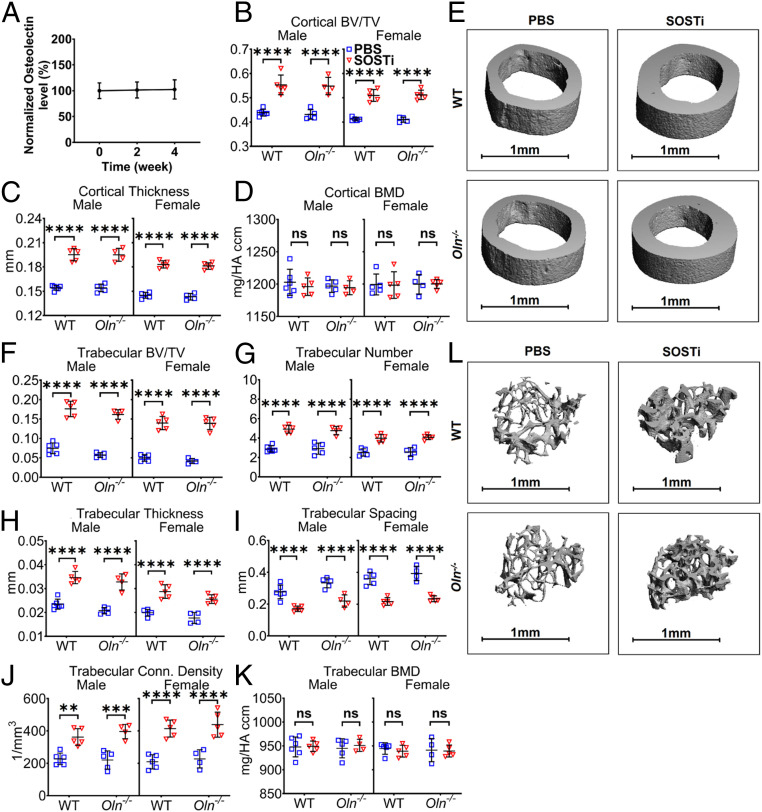
Fig. 3.
Osteolectin is not required for the effect of SOSTi on bone volume. (A) Serum osteolectin levels were measured in 2-mo-old wild-type (WT) mice treated with biweekly injections of SOSTi for 2 or 4 wk. Values in each mouse are presented as a percentage of baseline levels in the same mouse prior to treatment (n = 5 male and 5 female mice per group). (B–D) MicroCT analysis of the cortical bone volume/total volume (B), cortical thickness (C), and cortical bone mineral density (D) in the midfemur diaphysis of Osteolectin-deficient (Oln−/−) and control (WT) mice treated with PBS or SOSTi for 4 wk. (E) Representative microCT images of cortical bone in the midfemur diaphysis of male Oln−/− and control mice treated with PBS or SOSTi for 4 wk. (F–K) MicroCT analysis of the trabecular bone volume/total volume (F), trabecular number (G), trabecular thickness (H), trabecular spacing (I), trabecular connectivity density (J), and trabecular bone mineral density (K) in the distal femur metaphysis of Oln−/− and control mice treated with PBS or SOSTi for 4 wk. (L) Representative microCT images of trabecular bone in the distal femur metaphysis of male Oln−/− and control mice treated with PBS or SOSTi for 4 wk. B–K represent data from n = 4 to 6 male mice and 4 to 5 female mice per treatment. All data represent mean ± SD. Statistical significance was assessed using one-way ANOVAs followed by Dunnett’s multiple comparisons tests (A), three-way ANOVAs followed by Sidak’s multiple comparisons tests (B–D, F–I, and K), or one-way ANOVAs followed by Sidak’s multiple comparisons tests (J) (**P < 0.01; ***P < 0.001; ****P < 0.0001).