Abstract
Background:
Quantifying the degree to which spinal involvement of metastatic renal cell carcinoma (mRCC) is a locoregional phenomenon vs. a hematogenous, bone-specific affinity has implications for prognosis and antimetastatic therapy.
Objective:
To investigate the distribution of spinal metastasis in mRCC and to explore relationships between clinical factors and patterns of spinal spread.
Methods:
Patients with mRCC and spinal involvement from June 2005 to November 2018 were identified. Clinical and biologic features including primary tumor size and degree of spinal and nonbony metastatic involvement were collected. Spinal distributions were evaluated by the permutation test, with the null hypothesis that metastases are distributed uniformly across levels.
Results:
One hundred patients with 685 spinal levels involved by mRCC were evaluated. A nonuniform spatial distribution was observed across the cohort (P < 0.001); a preponderance of thoracolumbar involvement was noted with the mode at L3. No significant deviation in metastatic distribution from uniform was observed in right- or left-sided tumors, subgroups of distant or local metastases, or histology. Patients with smaller tumors (<4 cm) and local spread had distribution of spinal metastases not significantly different from uniform (P = 0.292 and P = 0.126, respectively).
Conclusions:
These data support a dominant locoregional as opposed to arterial hematogenous mechanism for early spinal dissemination of mRCC. Characterizations of the biologic molecular features contributing to osseous tropism and aggressive tumor biology (as seen in the subset of outlier patients with small tumors who appear to have more uniform spread), have implications for surveillance and are an area of active investigation.
Keywords: Metastatic distribution, Renal cell carcinoma, Spinal metastasis, Spine
1. Introduction
Bony metastasis is a severe and life-limiting complication of cancer, resulting in intractable pain, fractures, and limited mobility and is associated with earlier death in a variety of malignancies including renal cell carcinoma (RCC). Fully one-third of metastatic RCC (mRCC) patients develop bone metastases, and some one-third of these develop neurologic compromise owing to spinal cord or nerve root impingement [1,2]. Survival time is reduced to months in a variety of solid cancers over metastatic patients without bony involvement [2–4]. In addition to contributing to patient suffering and mortality, this disease imposes a significant societal burden: in a Nationwide Inpatient Sample analysis of 144,000 hospitalizations of RCC patients with bone metastasis, 20% involved skeletal-related events, and the cost of these visits increased 203% in inflation-adjusted terms from 1998 to 2010 [5].
Understanding mechanisms of metastasis may allow for development of improved prognostic tools for identifying patients at risk of this devastating complication, and ultimately of antimetastatic therapy. An example of this is the very poor prognosis correlated with acrometastasis in RCC [6]. The pathways involved in bony metastasis have yet to fully elucidated; VEGF and MET receptors have been shown to be expressed on osteoblasts and osteoclasts, regulating their proliferation, migration, and survival. The tyrosine-kinase inhibitor cabozantinib, with activity toward VEGF and MET has been postulated as a mediator of this process, and indeed, the clinical activity of cabozantinib in patients with bone metastases from RCC has been confirmed in a phase III trial [7–9].
One important way to understand this process, and whether there exist clinically meaningful bone-specific processes driving metastasis, is to differentiate the degree to which bony metastasis is a random stochastic event based on relative blood flow patterns, vs. driven by homing or organ-specific colonization ability. For example, the commonality of liver metastases in colon cancer is generally explained by portal drainage patterns, while liver metastases in uveal melanoma are to a large degree seen only with BAP1 mutation [10,11].
Quantifying distribution patterns of spinal metastases from the retroperitoneal space is a unique system for evaluating the degree to which bony distribution is a biologic capacity, vs. a locoregional phenomenon. In the former scenario, a uniform stochastic distribution of metastases would be anticipated across all evaluated bones, whereas in the latter scenario, peak metastatic seeding would present adjacent to the primary tumor mass. In RCC, thoracolumbar oligometastasis has been anecdotally observed to be common but has never been shown. In this study, we investigated the spatial distribution of metastases across vertebral levels in the first quantitative anatomic study of bone metastases.
2. Methods
Following institutional review board approval, an institutional database was queried to identify consecutive patients with a pathologic diagnosis of RCC and spinal metastatic involvement from June 2005 to November 2018 at a tertiary care center. Patients included were treatment-naïve, with de novo spinal metastases. Patient consent was not required as data was de-identified and collected in a retrospective fashion. A blinded radiologist examined the first cross-sectional imaging obtained involving the spine and scored each spinal level for absence or presence of disease. Clinical and biologic features including primary tumor size, histology, laterality, and degree of spinal and nonbony metastatic involvement (including regional lymph node and distant deposits) were retrieved from medical records. Patients with RCC without spinal involvement as well as patients with more than one malignancy were excluded. Likewise, patients with limited imaging data (only including central or only peripheral levels) were excluded, as they biased the analysis due to lack of coverage. These included 3 patients whose imaging covered C1–T11, T10–L5, and T7–S5. Data from remaining patients (n = 100) are presented and analysis was performed using by-level proportions with metastatic involvement among patients with imaging data at the given level. Data in the figures are presented in a normalized fashion to aid with visual interpretation.
2.1. Statistical analysis
Test for uniformity: Spinal metastatic distributions were evaluated by a permutation test, with the null hypothesis that rate spinal metastases are equal across all levels examined. Specifically, let Xj patients has a metastasis at level j among nj who had imaging on k-th level, j = 1,..29 consecutively ordered matching C1–C7, T1–T12, L1–L5, S1–S5. Let pjbe proportion of patients with metastasis at j-th level, estimated by . Let be the average observed metastasis rate per level. We define test statistic as . The null hypotheses is that p1 = … = p29. To generate the distribution of the proposed test statistic under the null hypothesis we use data specific number of metastases per patient observed in our study, defined Mi, i = 1,.., 100. Note that at most one metastasis per patient per level is counted, i.e., if multiple lesions are observed per level they are counted as 1. For each patient, at iteration b, we randomly allocate Mi metastases to levels 1,..,29 with equal probability. We then calculated the observed and the corresponding test statistic Tb. After repeating this for b = 1 1000 times we obtain the distribution of the test statistic under the null, and the P value is defined as . The advantage of the proposed test is that it takes into account the variation in the observed number of metastases across patient. All analyses were performed in the R platform v3.6.1. Statistical significance was assumed at P ≤ 0.05.
3. Results
One hundred patients with 685 spinal levels involved by mRCC were evaluated. Baseline clinical characteristics are presented in Table 1. Demographic and histologic features of this cohort demonstrated a preponderance of men (68%) and clear cell histologic subtype (71%). Sixty-two patients (62%) were imaged with MRI, 32 (32%) with CT, 3 (3%) with PET/CT, 2 (2%) with bone scan, and 1 (1%) with CT + MRI. Imaging capturing the entire length of the spine (C1–S5) was obtained in 67 patients (67%); the number of spinal levels involved across the cohort is detailed in Supplementary Table 1.
Table 1.
Baseline and clinical characteristicsa
| N = 100 | |
|---|---|
| Sex | |
| Male | 68 (68%) |
| Female | 32 (32%) |
| Histology | |
| Clear cell RCC | 71 (71%) |
| Clear cell RCC with sarcomatoid features | 12 (12%) |
| Chromophobe RCC | 15 (15%) |
| Collecting duct RCC | 1 (1.0%) |
| Unclassified RCC | 1 (1.0%) |
| Age at diagnosis (years) | 57 (19, 85) |
| Laterality | |
| Left | 55 (55%) |
| Right | 45 (45%) |
| Tumor size (cm) | 8.0 (1.2, 17.2) |
| Unknown | 5 |
| Metastatic site | |
| Distant | 28 (28%) |
| Distant + regional lymph nodes | 43 (43%) |
| Regional lymph nodes only | 12 (12%) |
| Spine only | 17 (17%) |
RCC = renal cell carcinoma.
Statistics presented: n (%); median (minimum, maximum).
Median tumor size was 8 cm (range 1.2–17.2 cm). With respect to locations of metastatic involvement, 71 patients (71%) were noted to have extra-osseous metastatic spread, and 29 patients (29%) had spine-only disease, with or without local retroperitoneal lymph node involvement. Thirty-five patients (35%) had metastatic involvement in only one vertebral level; 32 patients (32%) had involvement in 2 to 5 vertebral levels, and 33 patients (33%) had metastatic involvement in greater than 5 vertebral levels.
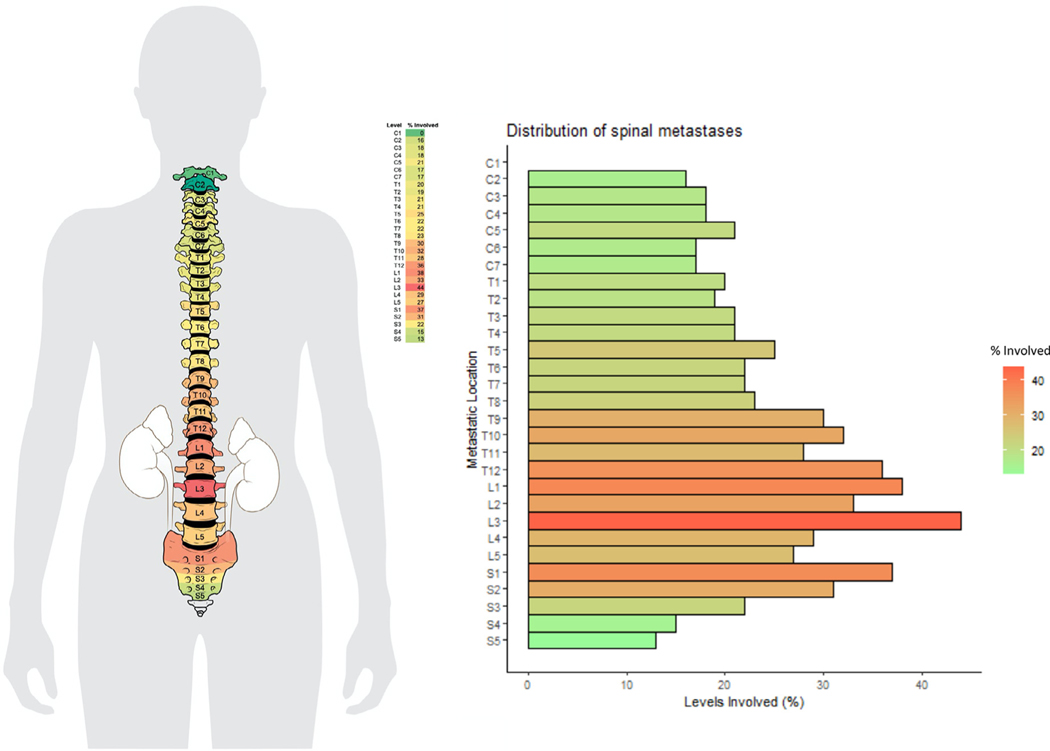
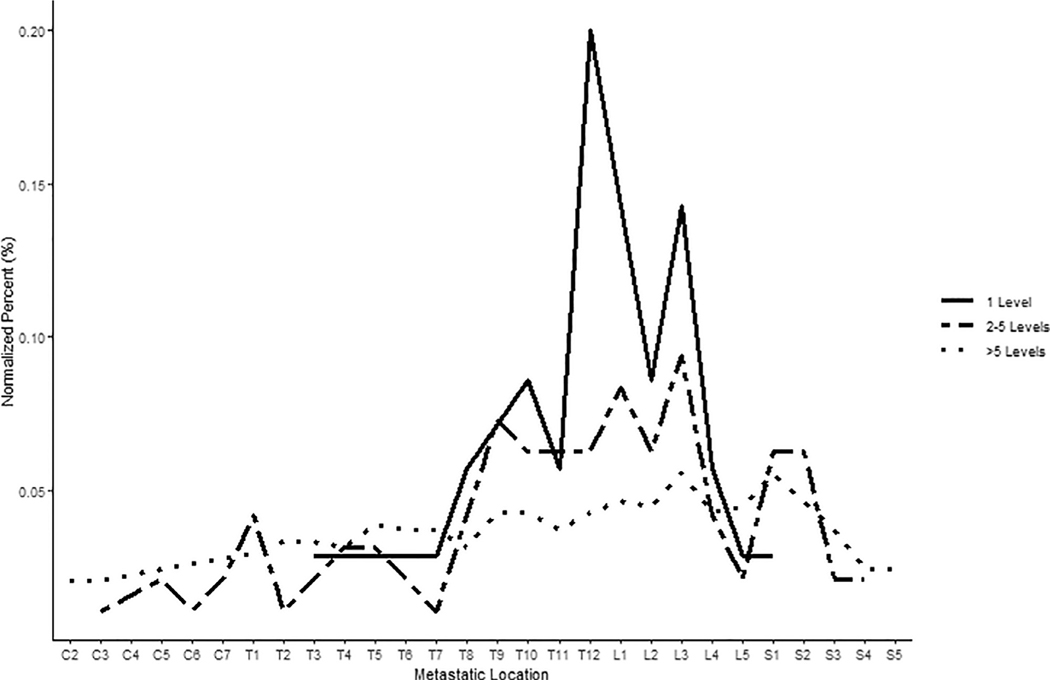
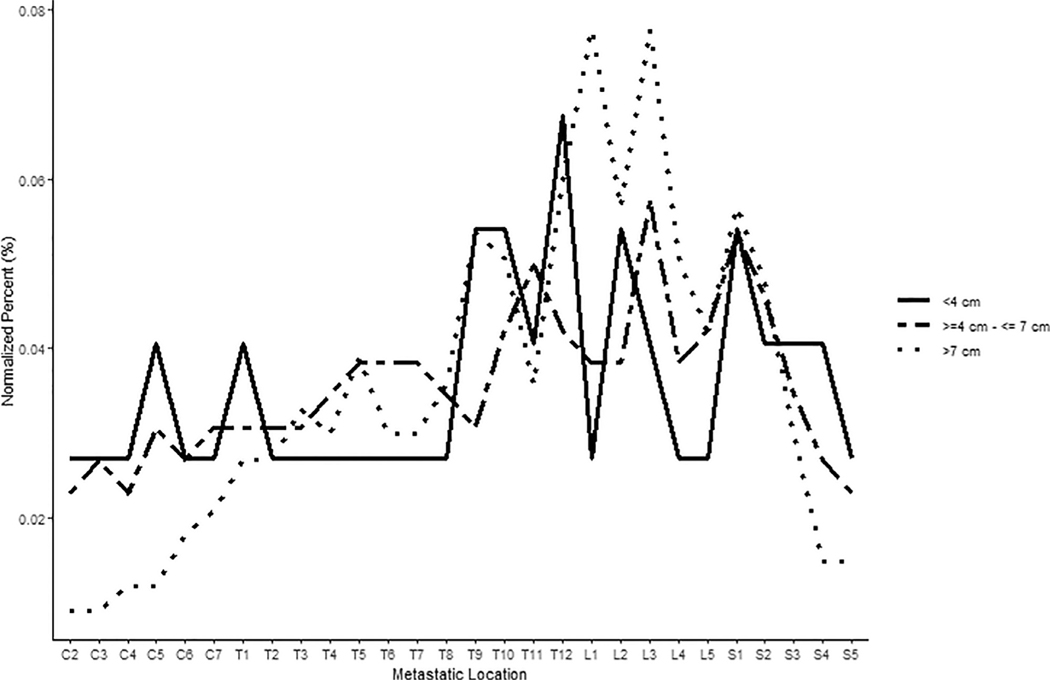
A heat map demonstrating the percentage of vertebral levels involved with metastatic RCC across all scanned vertebral levels is presented in Fig. 1, and a patient-level depiction of metastasis distribution is included in Supplementary Fig. 1. A nonuniform distribution of metastases by level was observed across the cohort (P < 0.001); a preponderance of thoracolumbar involvement was noted with the mode occurring at L3. Metastatic distribution was significantly nonuniform for right- or left-sided tumors, as well as for clear cell or nonclear cell renal cell histology (P < 0.001). Similar results were seen for patients with different number of levels involved with metastatic disease (1 level, P = 0.008; 2–5 levels, P = 0.004; >5 levels, P < 0.001; Fig. 2) and patients with both distant and local as well as distant only spread (both P < 0.001), while hypothesis of uniformity could not be rejected for patients with local spread (P = 0.126). Tumors <4 cm demonstrated distribution not significantly different from uniform (P = 0.292), unlike tumors 4 to 7 cm and >7 cm (both P < 0.001; Fig. 3). Note, however, the 2 groups were uniform distribution cannot be rejected are relatively small, with n = 12 patients each. We further explored the differences in uniformity between patients categorized into 3 groups: patients with spine-only disease, patients with spine and other bony (nonspinal) disease, and patients with spine and distant soft tissue metastases, finding no significant difference in uniformity between these 3 groups.
Fig. 1.
Heat map demonstrating the percentage of vertebral levels involved with metastatic renal cell carcinoma across all scanned vertebral level, and distribution of spinal metastasis across all patients (n = 100; mode = L3; p < 0.001).
Fig. 2.
Differences in spinal metastatic distribution by number of vertebral levels involved with metastatic renal cell carcinoma. Metastatic distribution was significantly non-uniform across all groups (1 level involved, p = 0.008; 2–5 levels involved, p = 0.004; >5 levels involved, p < 0.001).
Fig. 3.
Differences in spinal metastatic distribution by tumor size. Tumors <4 cm demonstrated spinal distribution not significantly different from uniform (p = 0.292), while tumors 4–7 cm and >7cm displayed significantly non-uniform distributions (both p < 0.001).
4. Discussion
In 1940, Oliver Batson described a large, valveless, venous network connecting the internal vertebral venous plexuses to the deep pelvic plexuses, purported to explain some degree of metastatic spread, vs. the widespread belief at the time that lymphatic drainage was the predominant metastatic route [12]. Ever since, this route has been suggested to be relevant in cases of thoracolumbar metastasis, particularly in cases of oligometastasis, but has never been shown to be the predominant mode of spinal spread [13,14]. Understanding this process with additional precision has basic biologic and potentially therapeutic implications. For example, the identification of RANKL-mediated osteoclast activation within the bone metastatic microenvironment has led to the successful adoption of RANK-directed therapy (denosumab and others) in several malignancies [15–18].
More importantly, the degree to which there exists bone specific capillary vs. venous extravasation this may allow for understanding whether there exist vascular bed-specific signaling. Furthermore, identification of tumor-specific predictors of additional metastasis and of survival would allow for treatment stratification. For example, many authors continue to advocate aggressive (and relatively morbid) surgical resection of solitary spine metastases specifically in the thoracolumbar junction on the basis of presumed favorable biology given the theoretical advantage of improved local control, despite effective radiation paradigms across histologies [19,20]. Surgical treatment of nonspinal bone metastases is also relied upon given poor radiosensitivity, and indeed aggressive surgery continues to be a mainstay for solitary disease in some centers [21,22].
In this study, we demonstrate a nonuniform distribution of RCC bone metastases centered at the upper/mid-lumbar spine immediately adjacent to the level of the kidneys, with a mode at L3, concordant with the hypothesis that RCC spread to the spine (and indeed early bony events) is largely driven by a locoregional mode of spread and not a random stochastic distribution to all bones within the body.
Furthermore, patients with spinal metastasis and clinical features associated with aggressive metastatic phenotypes demonstrated more equal rates of metastases across distribution of levels, suggesting inherent biologic features predisposing these tumors to widespread systemic dissemination through the whole-body circulation. These phenotypes included those with distant extraosseous spread and greater numbers of vertebral levels involved. In addition, we found support for our hypothesis that those with small primary tumor size (<4 cm) would paradoxically also have more equal rates of spread of metastases in the spinal distribution reflecting more aggressive metastatic capacity, as these tumors rarely metastasize [23]. Those harboring metastogenic cell populations despite their small size would thus be predicted to have aggressive metastatic capacity not reflecting the locoregional phenotype described above. The 4 cm cutoff is based on several series demonstrating differential survival and metastatic potential, resulting in the 2002 revision to RCC TNM guidelines [24–30].
This study demonstrates that spinal bony involvement in RCC is predominantly a function of distance from the kidneys and suggests that a circulating tumor concentration gradient biases metastatic deposit rates. This is concordant with the theory of the valveless Batson plexus acting as a conduit for spread from the retroperitoneal space to the vertebral column. Arterial spread, reflecting poor tumor deposit settling within locoregional compartments, would be predicted to be randomly distributed throughout spinal levels given this would involve admixture and random circulation of these metastogenic populations through the body. Future studies are geared towards an outlier analysis to define the molecular features that facilitate metastasis distant to the thoracolumbar junction.
An important limitation in our study design is its retrospective nature, and critically, that these data are captured at a single time point along each patient’s metastatic course. In other words, patients who have spine-only disease may progress to extra-spinal or soft tissue/visceral sites of metastases.
5. Conclusions
This analysis of bony distribution across spinal levels in bone-metastatic RCC supports the concept of a dominant locoregional mode of bone spread—in concordance with the theory of the Batson plexus serving as a conduit between the retroperitoneum and adjacent vertebral column—as opposed to stochastic studding of bones from an arterially diluted, hematogenous pool of tumor cells circulating throughout the body. Outlier analysis on metastatic deposits distant to the thoracolumbar junction, with diffuse spread to many bony levels, and those emanating from otherwise low-risk primary tumors, are being studied to characterize biologic molecular features contributing to aggressive osteo-tropic biology.
Supplementary Material
Acknowledgments
We wish to thank our patients and their caregivers.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.urolonc.2020.12.008.
Conflicts of interest
The authors have no disclosures relevant to this study.
References
- [1].Woodward E, Jagdev S, McParland L, et al. Skeletal complications and survival in renal cancer patients with bone metastases. Bone 2011;48(1):160–6. 10.1016/j.bone.2010.09.008. [DOI] [PubMed] [Google Scholar]
- [2].Santini D, Procopio G, Porta C, et al. Natural history of malignant bone disease in renal cancer: final results of an Italian bone metastasis surveyWilliams BO, ed PLoS One 2013;8(12):e83026. 10.1371/journal.pone.0083026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Sathiakumar N, Delzell E, Morrisey MA, et al. Mortality following bone metastasis and skeletal-related events among women with breast cancer: a population-based analysis of U.S. Medicare beneficiaries, 1999–2006. Breast Cancer Res Treat 2012;131(1):231–8. 10.1007/s10549-011-1721-x. [DOI] [PubMed] [Google Scholar]
- [4].Kuchuk M, Kuchuk I, Sabri E, Hutton B, Clemons M, Wheatley-Price P. The incidence and clinical impact of bone metastases in nonsmall cell lung cancer. Lung Cancer Amst Neth 2015;89(2):197–202. 10.1016/j.lungcan.2015.04.007. [DOI] [PubMed] [Google Scholar]
- [5].Antczak C, Trinh VQ, Sood A, et al. The health care burden of skeletal related events in patients with renal cell carcinoma and bone metastasis. J Urol 2014;191(6):1678–84. 10.1016/j.juro.2013.12.042. [DOI] [PubMed] [Google Scholar]
- [6].Stomeo D, Tulli A, Ziranu A, Perisano C, De Santis V, Maccauro G. Acrometastasis: a literature review. Eur Rev Med Pharmacol Sci 2015;19(15):2906–15. [PubMed] [Google Scholar]
- [7].Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373 (19):1814–23. 10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grano M, Galimi F, Zambonin G, et al. Hepatocyte growth factor is a coupling factor for osteoclasts and osteoblasts in vitro. Proc Natl Acad Sci U S A 1996;93(15):7644–8. 10.1073/pnas.93.15.7644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Knudsen BS, Gmyrek GA, Inra J, et al. High expression of the Met receptor in prostate cancer metastasis to bone. Urology 2002;60 (6):1113–7. 10.1016/s0090-4295(02)01954-4. [DOI] [PubMed] [Google Scholar]
- [10].Kalirai H, Dodson A, Faqir S, Damato BE, Coupland SE. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing. Br J Cancer 2014;111(7):1373–80. 10.1038/bjc.2014.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang J, Li S, Liu Y, Zhang C, Li H, Lai B. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: a population-based analysis. Cancer Med 2019. 10.1002/cam4.2673:cam4.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Batson OV. The function of the vertebral veins and their role in the spread of metastases. Ann Surg 1940;112(1):138–49. 10.1097/00000658-194007000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tosco L, Palazzetti A, Crivellaro S, Guaitoli P, Abbinante M, Frea B. Batson’s paravertebral venous plexus and single vertebral metastases from renal cell carcinoma. Urologia 2010;77(suppl 16):42–6. [PubMed] [Google Scholar]
- [14].Geldof AA. Models for cancer skeletal metastasis: a reappraisal of Batson’s plexus. Anticancer Res 1997;17(3A):1535–9. [PubMed] [Google Scholar]
- [15].Owari T, Miyake M, Nakai Y, et al. clinical features and risk factors of skeletal-related events in genitourinary cancer patients with bone metastasis: a retrospective analysis of prostate cancer, renal cell carcinoma, and urothelial carcinoma. Oncology 2018;95(3):170–8. 10.1159/000489218. [DOI] [PubMed] [Google Scholar]
- [16].Sahi C, Knox JJ, Clemons M, Joshua AM, Broom R. Renal cell carcinoma bone metastases: clinical advances. Ther Adv Med Oncol 2010;2(2):75–83. 10.1177/1758834009358417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mikami S, Katsube K, Oya M, et al. Increased RANKL expression is related to tumour migration and metastasis of renal cell carcinomas. J Pathol 2009;218(4):530–9. 10.1002/path.2567. [DOI] [PubMed] [Google Scholar]
- [18].Beuselinck B, Jean-Baptiste J, Couchy G, et al. RANK/OPG ratio of expression in primary clear-cell renal cell carcinoma is associated with bone metastasis and prognosis in patients treated with antiVEGFR-TKIs. Br J Cancer 2015;113(9):1313–22. 10.1038/bjc.2015.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kato S, Murakami H, Demura S, et al. Spinal metastasectomy of renal cell carcinoma: a 16-year single center experience with a minimum 3-year follow-up. J Surg Oncol 2016;113(5):587–92. 10.1002/jso.24186. [DOI] [PubMed] [Google Scholar]
- [20].Taunk NK, Spratt DE, Bilsky M, Yamada Y. Spine radiosurgery in the management of renal cell carcinoma metastases. J Natl Compr Cancer Netw JNCCN 2015;13(6):801–9. 10.6004/jnccn.2015.0093:; quiz 809. [DOI] [PubMed] [Google Scholar]
- [21].Laitinen M, Parry M, Ratasvuori M, et al. Survival and complications of skeletal reconstructions after surgical treatment of bony metastatic renal cell carcinoma. Eur J Surg Oncol 2015;41(7):886–92. 10.1016/j.ejso.2015.04.008. [DOI] [PubMed] [Google Scholar]
- [22].Baloch KG, Grimer RJ, Carter SR, Tillman RM. Radical surgery for the solitary bony metastasis from renal-cell carcinoma. J Bone Joint Surg Br 2000;82(1):62–7. 10.1302/0301-620x.82b1.9995. [DOI] [PubMed] [Google Scholar]
- [23].Daugherty M, Sedaghatpour D, Shapiro O, Vourganti S, Kutikov A, Bratslavsky G. The metastatic potential of renal tumors: influence of histologic subtypes on definition of small renal masses, risk stratification, and future active surveillance protocols. Urol Oncol 2017;35(4). 10.1016/j.urolonc.2016.11.009:153.e15–153.e20. [DOI] [PubMed] [Google Scholar]
- [24].Lerner SE, Hawkins CA, Blute ML, et al. Disease outcome in patients with low stage renal cell carcinoma treated with nephron sparing or radical surgery. J Urol 1996;155(6):1868–73. [PubMed] [Google Scholar]
- [25].Hafez KS, Novick AC, Campbell SC. Patterns of tumor recurrence and guidelines for follow-up after nephron sparing surgery for sporadic renal cell carcinoma. J Urol 1997;157(6):2067–70. [PubMed] [Google Scholar]
- [26].Belldegrun A, Tsui KH, deKernion JB, Smith RB. Efficacy of nephron-sparing surgery for renal cell carcinoma: analysis based on the new 1997 tumor-node-metastasis staging system. J Clin Oncol 1999;17(9):2868–75. 10.1200/JCO.1999.17.9.2868. [DOI] [PubMed] [Google Scholar]
- [27].Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm or less in a contemporary cohort. J Urol 2000;163(3):730–6. [PubMed] [Google Scholar]
- [28].Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year follow-up. J Urol 2000;163(2):442–5. [PubMed] [Google Scholar]
- [29].Becker F, Siemer S, Humke U, Hack M, Ziegler M, Stöckle M. Elective nephron sparing surgery should become standard treatment for small unilateral renal cell carcinoma: long-term survival data of 216 patients. Eur Urol 2006;49(2):308–13. 10.1016/j.eur-uro.2005.10.020. [DOI] [PubMed] [Google Scholar]
- [30].Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. John Wiley & Sons; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.