Abstract
Detecting shifts in trait values among populations of an invasive plant is important for assessing invasion risks and predicting future spread. Although a growing number of studies suggest that the dispersal propensity of invasive plants increases during range expansion, there has been relatively little attention paid to dispersal patterns along elevational gradients. In this study, we tested the differentiation of dispersal-related traits in an invasive plant, Galinsoga quadriradiata, across populations at different elevations in the Qinling and Bashan Mountains in central China. Seed mass–area ratio (MAR), an important seed dispersal-related trait, of 45 populations from along an elevational gradient was measured, and genetic variation of 23 populations was quantified using inter-simple sequence repeat (ISSR) markers. Individuals from four populations were then planted in a greenhouse to compare their performance under shared conditions. Changing patterns of seed dispersal-related traits and populations genetic diversity along elevation were tested using linear regression. Mass–area ratio of G. quadriradiata increased, while genetic diversity decreased with elevation in the field survey. In the greenhouse, populations of G. quadriradiata sourced from different elevations showed a difference response of MAR. These results suggest that although rapid evolution may contribute to the range expansion of G. quadriradiata in mountain ranges, dispersal-related traits will also likely be affected by phenotypic plasticity. This challenges the common argument that dispersal ability of invasive plants increases along dispersal routes. Furthermore, our results suggest that high-altitude populations would be more effective at seed dispersal once they continue to expand their range downslope on the other side. Our experiment provides novel evidence that the spread of these high-altitude populations may be more likely than previously theorized and that they should thus be cautiously monitored.
Keywords: Dispersal-related traits, elevation gradient, genetic variation, invasive plant, population differentiation
In this study, we tested the differentiation of dispersal-related traits in an invasive plant Galinsoga quadriradiata across populations at different elevations in Qinling and Bashan Mountains, China. Our results suggest that the dispersal ability of G. quadriradiata decreases along elevational dispersal routes as a result of genetically based rapid evolution and phenotypic plasticity, challenging the common argument that dispersal ability of invasive plants increases along dispersal routes. Our findings provide new insight into how the dispersal ability of invasive plants may change along the elevational gradient. This is important for revealing the upward expansion dynamic of invasive plants in high mountains.
Introduction
Invasive plants, which tend to spread uncontrollably and cause environmental or economic damage (Rejmánek et al. 2013), are increasingly colonizing into high-altitude areas of mountain ranges as a consequence of climate change (Alexander et al. 2016, 2018), land-use transformations and anthropogenic disturbances (Carboni et al. 2018). However, there has been relatively little attention paid to how mountains affect the upward expansion of invasive plants. It is often suggested that high mountain ranges are less likely to be invaded due to stressful abiotic environments (Körner 2007). Compared to lower altitude, plants are exposed to lower temperatures and shorter growing seasons in high-altitude area (Watermann et al. 2019), and soil nutrients may also be less abundant (Soethe et al. 2008; Drollinger et al. 2017). The importance of these factors is compounded by the fact that conditions can drastically change over a relatively small range of altitude (Becker et al. 2005). Invasive plants must therefore be able to acclimate to very different environments over small distances to move upslope.
Plants have been shown to do this via two main strategies: phenotypic plasticity and genetic adaptation (Liu et al. 2016a). Phenotypic plasticity refers to the ability of invasive plants to adjust their phenotype along environmental gradients and is important for supporting population persistence and continued spread across elevations (Alexander et al. 2016). Invasive plants usually demonstrate higher phenotypic plasticity compared to native species, which has been linked to their expansions in variable environmental conditions (Davidson et al. 2011; LaForgia et al. 2020). Phenotypic plasticity is typically important when environmental conditions are highly variable because it allows plants to acclimate within a relatively short timescale. Plant biomass allocation, height and number of flowers all demonstrate some degree of plasticity associated with elevation in mountain ranges (Frei et al. 2014; Ensslin and Fischer 2015). Other examples include leaf mass per area and leaf density, which usually decrease with elevation, while leaf thickness increases with elevation (Vitasse et al. 2014). It has thus been suggested that the invasion of some invasive plants benefits from phenotypic plasticity when expanding into higher elevations (Liao et al. 2016; Bustamante et al. 2018).
In contrast, genetic adaptation is more important over long timescales and occurs as a result of rapid evolution under novel natural selection pressure. Genetic differentiation among populations is an expected signal of adaptive response to changing environmental factors (Volis et al. 2015). Therefore, studies of population-level genetic differentiation of invasive species could offer insights into mechanisms of invasions (Ueno et al. 2015). For example, genetic differentiation may change during the expansion of invasive plants into high elevation (Bustamante et al. 2018), as has been observed for Solidago canadensis, for which genetic diversity decreased significantly with elevation (Alexander et al. 2009).
Although these mechanisms are likely to affect many traits related to plant fitness, range expansion is likely to be most affected by shifts in traits related to dispersal (Monty and Mahy 2010; Hargreaves et al. 2014; Huang et al. 2015; Tabassum and Leishman 2017). Spatial selection theory suggests that plants are sorted through space according to their dispersal ability or investment in dispersal (Thomson et al. 2018), and subsequent research has shown that individuals with greater dispersal ability accumulate at expansion range edges (Phillips et al. 2010; Shine et al. 2011; Andrade-Restrepo et al. 2019). This also implies that individuals with lower dispersal ability would form high-density populations in the center of the range. This is further supported by evidence showing that invasive plants usually experience decreased intraspecific competition and have high preadaptation ability at the invasion edge associated with low conspecific density (Alzate et al. 2020). Therefore, marginal populations with high dispersal ability will likely aggravate invasion (Phillips et al. 2010; Seale and Nakayama 2020). Moreover, high levels of dispersal can reduce the probability of inbreeding, thereby reducing the occurrence of deleterious genetic effects that might otherwise hinder invasion (Monty and Mahy 2010; Williams et al. 2019).
It is therefore expected that variation in traits related to dispersal will be found along the invasion routes of exotic plants (Rejmanek and Richardson 1996; Davidson et al. 2011; Kanapeckas et al. 2018), with traits associated with greater dispersal ability found at the invasive edge of the range. Detecting shifts in population-level traits of invasive plant species is thus an important tool for assessing invasion risks and predicting future spread (Estrada et al. 2016; Laeseke et al. 2020). However, few studies have tested whether the dispersal-related traits of invasive plants change along elevational gradients, and, if so, whether it is due to phenotypic plasticity or due to genetic adaptation (Monty and Mahy 2010; Steyn et al. 2017). Determining the source of the change in dispersal-related traits is important because evidence for phenotypic plasticity would indicate a greater risk of invasion associated with a higher ability to quickly acclimate to diverse environmental conditions, whereas evidence for genetic variation would instead suggest that a greater risk of invasion associated with a higher genetic diversity.
In this study, we investigated how dispersal-related traits of invasive Galinsoga quadriradiata vary along an elevational gradient in a high mountain range in central China, and whether such changes are attributable to genetic differentiation or phenotypic plasticity. Specifically, we addressed the following questions: (i) How do population-level traits of G. quadriradiata related to dispersal vary across an elevational gradient? (ii) Are these changes attributable to phenotypic plasticity, genetic adaptation or a combination of both? (iii) What do these changes suggest about the future expansion of G. quadriradiata in the mountains of central China?
Materials and Methods
Study organism
Galinsoga quadriradiata (Asterales: Asteraceae) is an annual herbaceous plant originating in Central and South America. It is a harmful agricultural invasive weed, mainly established in moist, warm temperate and subtropical zones around the world on abandoned land or farmland (Liu et al. 2016b). It can reduce agricultural production by approximately 50 % (Kabuce and Priede 2010). Since its first report on Lushan Mountain in Jiangxi Province in 1979, it has been found across all suitable climatic areas in China (Yang et al. 2018). Due to a lack of competitive and colonization ability in natural communities, it is usually considered a relatively weak invader (Liu et al. 2018). However, its influences on natural and agricultural ecosystems are significant due to its considerably large seed yield (approximately 46 000 fertile seeds per square meter) and high dispersal potential attributed to light seed mass which covered with hairs (Liu et al. 2016b). Galinsoga quadriradiata has recently started to expand towards the northern and western regions of China, which are at relatively high altitudes (Yang et al. 2018). This includes the Qinling and Bashan Mountains, high peaks that act as major barriers preventing the further dispersal of exotic species from the coastal areas of eastern and southern China. Large populations are already widely established on the southern slopes, threatening the conservation of biodiversity as well as agricultural production in this region (Liu et al. 2016b).
Study location
The Qinling and Bashan mountain ranges in central and south-western China (30˚5'–34˚59'N, 102˚54'–112˚4'E, about 222 300 km2) are characterized by complex topography and distinct environmental conditions between the northern and southern regions (Wang et al. 2017). The highest peaks in the Qinling and Bashan Mountains are at 3767 and 3105 meters above sea level (m asl), respectively. The mountains serve as natural barriers between the southern subtropical and the northern warm temperate regions of central China, and also as a boundary between the Palearctic and Oriental Regions in eastern Asia. In the Qinling Mountains, areas lower than 1000 m asl on south-facing slopes are dominated by a subtropical climate while the areas above 1000 m asl on south-facing slopes and all elevations on north-facing slopes are more temperate. The Bashan Mountains are approximately 50 km south of the Qinling Mountains, extend from east to west in parallel with them (Zhan et al. 2009), and are dominated by a subtropical climate. In the Qinling Mountains, the average annual temperature is 11–13 °C and the average annual precipitation is between 590 and 764 mm (Zhao et al. 2014). The average annual temperature in the Bashan Mountains ranges from 14.5 to 16.5 °C, and average annual precipitation is between 800 and 1400 mm (Li et al. 1990).
Field survey
In July 2015, we conducted a field survey across both mountain ranges. In total, 45 populations of G. quadriradiata were surveyed along an elevational gradient ranging from 220 to 2128 m asl (Fig. 1; Table 1). Each population was separated by at least 150 m in altitude. In each population, we randomly chose 4–20 mature individuals to collect seed and leaf samples from. The number of mature seeds per capitulum (hereafter NSC) was counted after randomly choosing at least 5 capitula per individual. All seeds were air-dried and loosely stored in envelopes at 4 °C until measurement. We also collected approximately 8–16 young leaves per plant. The collected leaves were quickly dried in a plastic bag with silica gel and then stored in a −80 °C freezer until use.
Figure 1.
Map of the elevational populations of G. quadriradiata investigated in the Qinling and Bashan Mountains.
Table 1.
The locations of the elevational populations of Galinsoga quadriradiata. The last column showed the populations used for genetic diversity analysis.
| Population | Longitude (°) | Latitude (°) | Elevation (m) | Mountains | Genetic |
|---|---|---|---|---|---|
| BS1 | 108.98 | 32.65 | 223 | Bashan | |
| BS2 | 108.88 | 32.49 | 370 | Bashan | Y |
| BS3 | 108.94 | 32.31 | 436 | Bashan | Y |
| BS4 | 108.98 | 32.27 | 504 | Bashan | Y |
| BS5 | 109.01 | 32.22 | 646 | Bashan | Y |
| BS6 | 109.11 | 32.14 | 691 | Bashan | |
| BS7 | 109.19 | 32.11 | 859 | Bashan | Y |
| BS8 | 109.22 | 32.1 | 890 | Bashan | Y |
| BS9 | 109.49 | 31.89 | 1005 | Bashan | Y |
| BS10 | 109.28 | 32.07 | 1185 | Bashan | Y |
| BS11 | 109.42 | 31.92 | 1248 | Bashan | Y |
| BS12 | 109.41 | 31.93 | 1321 | Bashan | Y |
| BS13 | 109.31 | 32.04 | 1513 | Bashan | Y |
| BS14 | 109.38 | 31.97 | 1594 | Bashan | Y |
| BS15 | 109.32 | 32.03 | 1756 | Bashan | Y |
| BS16 | 109.33 | 32.02 | 1947 | Bashan | Y |
| QL1 | 108.21 | 33.09 | 414 | Qinling | Y |
| QL2 | 108.08 | 33.27 | 523 | Qinling | |
| QL3 | 108.82 | 34.02 | 565 | Qinling | |
| QL4 | 108.19 | 33.3 | 581 | Qinling | Y |
| QL5 | 108.28 | 33.31 | 680 | Qinling | |
| QL6 | 108.32 | 33.35 | 829 | Qinling | Y |
| QL7 | 107.35 | 33.66 | 839 | Qinling | |
| QL8 | 107.31 | 34.25 | 855 | Qinling | |
| QL9 | 108.04 | 33.88 | 860 | Qinling | |
| QL10 | 106.94 | 33.61 | 896 | Qinling | |
| QL11 | 108.35 | 33.38 | 916 | Qinling | |
| QL12 | 107.4 | 33.72 | 985 | Qinling | |
| QL13 | 107.54 | 33.59 | 1041 | Qinling | |
| QL14 | 108.01 | 33.83 | 1048 | Qinling | Y |
| QL15 | 108.72 | 33.76 | 1060 | Qinling | Y |
| QL16 | 107.12 | 33.88 | 1097 | Qinling | |
| QL17 | 108.61 | 33.56 | 1163 | Qinling | |
| QL18 | 108.4 | 33.41 | 1203 | Qinling | Y |
| QL19 | 108.58 | 33.57 | 1229 | Qinling | |
| QL20 | 108.78 | 33.77 | 1236 | Qinling | Y |
| QL21 | 108.41 | 33.42 | 1262 | Qinling | |
| QL22 | 108.42 | 33.43 | 1307 | Qinling | |
| QL23 | 108.62 | 33.57 | 1343 | Qinling | |
| QL24 | 108.54 | 33.54 | 1349 | Qinling | Y |
| QL25 | 107.26 | 34.04 | 1454 | Qinling | |
| QL26 | 108.45 | 33.43 | 1563 | Qinling | Y |
| QL27 | 108.79 | 33.83 | 1725 | Qinling | |
| QL28 | 108.5 | 33.47 | 2080 | Qinling | |
| QL29 | 108.49 | 33.47 | 2128 | Qinling |
Measurement of dispersal-related traits
For each plant from the field survey, we calculated NSC and measured the combined weight of one hundred seeds (HSW). To calculate HSW, we randomly selected 100 ripe seeds from the collected seeds of each population and weighted them. At least five replicates were made for each population. We used WinSEEDLE Pro (WinSEEDLE™, Régent Instruments Inc., Québec, QC, Canada) to measure seed length, pappus length and pappus width. Each time, we randomly selected 30 ripe seeds from an elevational population and scanned and analysed them. We repeated this 10 times for each population.
Dispersal ability of wind-dispersed Asteraceae diaspores, such as G. quadriradiata, is typically approximated by morphological characteristics (Matlack 1987; Monty and Mahy 2010; Seale and Nakayama 2020). Here, we used plume loading (mass–area ratio, MAR), a morphological characteristic commonly associated with dispersal ability (Huang et al. 2015). Mass–area ratio (MAR) can be calculated as
where m is the seed mass and R is the pappus radius which is calculated as half of the pappus width. Mass–area ratio has been shown to be a reliable indicator of the dispersal ability of other wind-dispersed Asteraceae (Matlack 1987; Meyer and Carlson 2001), so it should also be representative of dispersal ability of our study species. Low MAR values (i.e. lighter seed with larger pappus) therefore indicate greater dispersal potential (Augspurger and Franson 1987).
Genetic differentiation
We used the inter-simple sequence repeat (ISSR) markers to analyse the genetic diversity of G. quadriradiata populations. Total DNA was isolated using the CTAB method (Gawel and Jarret 1991), and diluted in sterilized Millipore water. Inter-simple sequence repeat primers were synthesized (by Shanghai Sangon Biological Engineering Technology & Service Co., Ltd) according to the primer set published by the University of British Columbia (UBC) (http://www.michaelsmith.ubc.ca/services/NAPS/Primer_Sets/Primers_Oct2006.pdf). Seven primers (UBC 816, UBC 817, UBC 818, UBC 826, UBC 847, UBC 855 and UBC 857) were used to amplify the 233 samples from 23 of the 45 populations. The polymerase chain reaction (PCR) system for ISSR analysis was as follows: total volume 25 µL; 12.5 μL 2 × Es Taq MasterMix (Dye), 1 μL 10 μmol L−1 primer, 1 μL 50 ng μL−1 DNA, 10.5 μL ddH2O. All reactions were performed on a gradient PCR instrument (Agilent SureCycler 8800). The PCR procedure was as follows: pre-degeneration at 94 °C for 2 min, degeneration at 94 °C for 30 s, annealing at 50.5–55 °C for 30 s (annealing temperature depends on different primer), extension at 72 °C for 1 min, 6 cycles; degeneration at 94 °C for 30 s, annealing at 50.5–55 °C for 30 s (annealing temperature depends on different primer), extension at 72 °C for 1 min, 32 cycles; final extension at 72 °C for 3 min and preservation at 4 °C. The amplification products were separated in 2 % agarose gel (containing 0.5 mg mL−1 ethidium bromide) in 1 TBE (Tris-Borate-EDTA), and the separated bands were visualized under UV light by using an Electrophoresis Documentation and Analysis System 120 (Eastman Kodak Company). DL2000 ladder (Dongsheng Biotech Ltd) was used as DNA molecular weight markers.
Greenhouse experiment
To explore the influence of genetic differentiation on dispersal ability of different populations in a common environment on the basis of field survey, we randomly selected populations from four elevations. In late April 2016, seeds from the four populations (BS1 (223 m asl), QL5 (680 m asl), QL22 (1307 m asl) and BS15 (1756 m asl); Table 1) were sown in nursery pots in a greenhouse (at 430 m asl) located on a plain on the northern side of the Qinling Mountains. The environmental condition of the QL5 population was most similar to that of the greenhouse. Three weeks later, when the seedlings were approximately 5 cm in height, they were carefully transplanted into plastic pots (diameter 12 cm, height 10 cm). The pots were filled with a 1:1 mixture of soil and sand using soil collected from a forest plantation next to the greenhouse. The total nitrogen and total phosphorus concentrations of the mixed soil were 3.02 and 0.65 mg g−1, respectively. Eight replicate plants from each population were grown, yielding a total of 32 pots. Pots were randomly arranged in an 80-m2 greenhouse, and the positions of the pots were changed at random every 2 weeks. Seedlings were watered daily and were replaced if mortality occurred within 1 week of the start of the experiment. The plants began to bloom in early June and the seeds began to disperse in late June. We collected mature seeds every 2 days until all plants no longer yielded seeds. All seeds were stored in envelopes and air-dried under laboratory conditions until measurement. We then analysed seed dispersal-related traits using the same method as described above for the field survey.
Data analyses
We constructed linear mixed-effects models to evaluate the effects of elevation on diaspore-related traits (HSW, NSC, seed length, pappus length, pappus width and MAR), using the packages ‘lme4’ and ‘lmerTest’ in R-3.5.3 (R Development Core Team 2019). Elevation and mountain (Qinling and Bashan) were used as the fixed and random factors, respectively, in the model.
Only distinct, reproducible and well-resolved PCR fragments were included in the statistical analysis for genetic differentiation. Inter-simple sequence repeat bands were scored as presence (1) or absence (0) characters, to construct the binary matrix. To compare the amount of total genetic variation partitioned within and among populations, three methods were employed, namely the hierarchical analysis of molecular variances (AMOVA), the analysis of Shannon’s diversity (Qian and Ge 2001) and Nei’s analysis of genetic diversity (Nei 1987). Genetic data were analysed in GenAlEx 6.502 (Peakall and Smouse 2012). Genetic diversity (i.e. the percentage of polymorphic loci, the Shannon’s information index and the expected heterozygosity) was calculated. The percentage of polymorphic loci (PPL) is an indicator of genetic polymorphism, with higher values indicating a higher proportion of polymorphic loci, and thus greater genetic variability. The Shannon’s information index (I) is usually used as an evaluation index, with higher values reflecting higher genetic diversity. Expected heterozygosity (He) is often used to measure the genetic diversity of a population, with higher values indicating richer the genetic diversity.
Linear mixed-effect models were then constructed to test for associations between genetic diversity and elevation (fixed factor) for the field seedlings, using mountain (Qinling and Bashan) as a random factor. We then constructed the phylogenetic tree of UPGMA based on Nei’s genetic distance using MEGA-X (Kumar et al. 2018). For the greenhouse experiment, we performed a linear regression analysis to evaluate the effects of elevation on the four dispersal-related traits (seed length, pappus length, pappus width and MAR) using the package ‘multcomp’ in R-3.5.3 (R Development Core Team 2019). We also performed a one-way ANOVA analyses and Tukey’s multiple comparison tests to compare the MAR of the four populations in the greenhouse experiment and the field survey.
Results
Phenotypic trait variation in the field survey
The results of the linear mixed-effect models revealed that elevation has significant effects on HSW, NSC, seed length, pappus length, pappus width and MAR (Table 2; seeSupporting Information—Table S1). HSW (Fig. 2A), NSC (Fig. 2B), seed length (Fig. 2C) and MAR (Fig. 2F) showed significant positive correlations with elevation while pappus length (Fig. 2D) and pappus width (Fig. 2E) were significantly negatively correlated with elevation.
Table 2.
The results of linear mixed-effect models on MAR, HSW (weight of one hundred seeds), NSC (number of mature seed per capitulum), seed length, pappus length and pappus width using elevation and mountain as fixed and random factors, respectively. *P < 0.05; **P < 0.01; ***P < 0.001; nsP > 0.05.
| Dependent variable | Fixed effect | Estimate | SE | t | P | Signif. Codes |
|---|---|---|---|---|---|---|
| MAR | Intercept | 0.006 | 1.35 × 10–4 | 44.753 | <0.001 | *** |
| Elevation | 8.83 × 10–7 | 9.27 × 10–8 | 9.526 | <0.001 | *** | |
| HSW | Intercept | 0.018 | 6.94 × 10–4 | 25.46 | 0.02 | * |
| Elevation | 2.65 × 10–6 | 1.05 × 10–7 | 25.15 | <0.001 | *** | |
| NSC | Intercept | 18.59 | 1.096 | 16.961 | <0.001 | *** |
| Elevation | 0.002 | 9.18 × 10–4 | 2.318 | 0.0231 | * | |
| Seed length | Intercept | 1.382 | 0.016 | 84.45 | 0.004 | ** |
| Elevation | 2.27 × 10–5 | 3.50 × 10–6 | 6.48 | <0.001 | *** | |
| Pappus length | Intercept | 1.249 | 0.039 | 32.3 | 0.0143 | * |
| Elevation | –1.4 × 10–4 | 6.79 × 10–6 | –20.69 | <0.001 | *** | |
| Pappus width | Intercept | 2.061 | 0.04 | 50.969 | 0.003 | ** |
| Elevation | –2.75 × 10–5 | 1.25 × 10–5 | –2.193 | 0.028 | * |
Figure 2.
The linear mixed-effect model of seed dispersal-related traits (HSW, NSC, seed length, pappus length, pappus width and MAR) and elevation in the field survey. Fixed factor, Elevation; random factor, Mountain. HSW, the weight of one hundred seeds; NSC, the number of mature seed per capitulum; MAR, mass–area ratio.
Genetic diversity
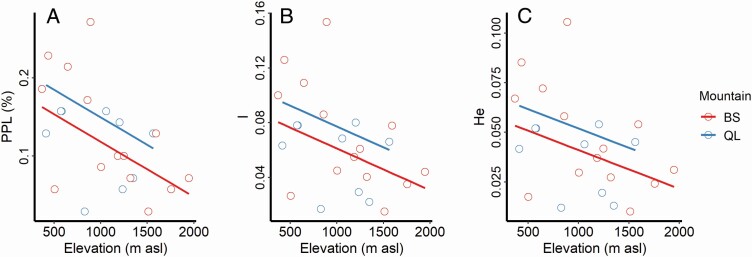
A total of 70 polymorphic loci were obtained from the ISSR analysis. Genetic variation between populations accounted for 81 % of total genetic variation while intrapopulation variation accounted for the remaining 19 % (Table 3). PPL, I and He were significantly negatively correlated with population elevation (Fig. 3; Table 4; seeSupporting Information—Table S2), indicating that genetic diversity decreases at higher altitudes.
Table 3.
The result of AMOVA analysis for evaluating genetic variation from among and within populations. The AMOVA analysis was significant after 999 permutation tests (PhiPT = 0.809, P = 0.001).
| Source | df | SS | MS | Est. Var. | % |
|---|---|---|---|---|---|
| Among pops | 22 | 1449.225 | 65.874 | 6.368 | 81 % |
| Within pops | 210 | 316.431 | 1.507 | 1.507 | 19 % |
| Total | 232 | 1765.657 | 7.875 | 100 % |
SS, sum of squared deviation; MS, mean square deviation.
Figure 3.
The linear mixed-effect model of genetic diversity (PPL, I and He) and the elevation of the field population. Fixed factor, Elevation; random factor, Mountain. PPL, percentage of polymorphic loci (%); I, Shannon’s information index; He, expected heterozygosity.
Table 4.
The results of linear mixed-effect models on PPL, I and He using elevation and mountain as fixed and random factors, respectively. *P < 0.05; **P < 0.01; ***P < 0.001; nsP > 0.05.
| Dependent variable | Fixed effect | Estimate | SE | t | P | Signif. codes |
|---|---|---|---|---|---|---|
| PPL | Intercept | 0.204 | 0.016 | 12.93 | 0.046 | * |
| Elevation | −7.05 × 10–5 | 1.46 × 10–6 | –48.47 | <0.001 | *** | |
| I | Intercept | 0.1 | 0.008 | 12.28 | 0.048 | * |
| Elevation | −3.05 × 10–5 | 7.88 × 10–7 | –38.71 | <0.001 | *** | |
| H e | Intercept | 0.066 | 0.005 | 12.05 | 0.049 | * |
| Elevation | −1.95 × 10–5 | 5.44 × 10–7 | –35.91 | <0.001 | *** |
The sum of the branch length of the optimal phylogenetic tree was 1.32 (Fig. 4). Populations were genetically clustered into two groups, each of which not only contained sites from both mountains, but also contained sites spanning multiple elevations. Smaller population clusters were typically composed of populations that had similar elevations or that were geographically closed to each other.
Figure 4.
UPGMA cluster analysis of 23 populations of G. quadriradiata based on Nei’s genetic distances.
Greenhouse experiment
The interpopulation trends of dispersal-related traits described above for the field survey were differed substantially from the results from the greenhouse study. Seed length (Fig. 5A), pappus length (Fig. 5B) and MAR (Fig. 5D) were significantly negatively correlated with the elevation of the seed source population. Pappus width was not significantly correlated with the elevation of the seed source population (Fig. 5C).
Figure 5.
The linear relationship between seed dispersal-related traits and the elevation of the source population in the greenhouse experiment. MAR, seed mass–area ratio.
Discussion
Elevational variation associated with seed dispersal-related traits of invasive species has not yet been fully explored, creating a knowledge gap of how phenotypic plasticity and genetic diversity affect the invasive success of plants at high altitudes. In this study, we examined the phenotypic plasticity and genetic diversity of dispersal-related traits in invasive G. quadriradiata in the mountains of central China, and investigated whether dispersal-related traits variation in this species is attributable to genetic differentiation, plasticity or some combination thereof. We found that many trait values were significantly associated with elevation in our field survey, with plants from higher-elevation populations tending to have larger seeds with smaller pappi (feathery protusions which aid in dispersal). Furthermore, these trends were associated with reductions in genetic diversity at high elevations, suggesting that adaptation is largely responsible for the observed patterns. However, some of contradicted results from our greenhouse study showed greater dispersal-related traits of higher-elevation population in unstressed environment, suggesting that trait plasticity could still play an important role in the range expansion of invasive species in mountain ranges.
The clinal trend of dispersal-related traits along the elevational route
Spatial selection theory hypothesizes that dispersal phenotypes are spatially separated and that only the best dispersers will accumulate towards the range front (Shine et al. 2011). Evidence to support this theory has been demonstrated in many invasive plants (Monty and Mahy 2010; Huang et al. 2015) as well as in evolutionary simulation models (Dytham 2009). This theory is also consistent with genetic differentiation theory (Travis and Dytham 2002; Alford et al. 2009; Andrade-Restrepo et al. 2019), which predicts high dispersal ability in individuals near the forward edge of the expanding range. In mountain ranges, where the range edge is at high elevation, we would thus expect to find correlations between dispersal ability and elevation.
For wind-dispersed Asteraceae seeds, dispersal ability is often approximated by measuring seed MAR, which is correlated with terminal seed velocity (Monty et al. 2008; Monty and Mahy 2010; Seale and Nakayama 2020). Higher MAR values have typically been found to indicate lower dispersal ability (Huang et al. 2015), so, following spatial selection theory, one would expect that invasive plants at high elevation should have lower MAR than those closer to the center of their range.
We found the opposite trend in our field survey, with G. quadriradiata seeds exhibiting positive correlations between MAR and elevation (Fig. 2F) and negative correlations between elevation and traits related to pappus size (Fig. 2D and E). In contrast to spatial selection theory, this suggests that this species has a diminished dispersal ability at high elevations. If this is true, it would mean that mountain ranges slow the invasion of G. quadriradiata by negatively affecting dispersal, a novel finding for high-elevation invasion dynamics.
Still, it is important to note these trends could instead indicate that low MAR and large pappi are not as advantageous in high-elevation environments as they are in other systems. For example, increased wind strength and occurrence of updrafts associated with mountain ranges might allow plants to achieve the same dispersal distance with relatively smaller investments in dispersal-related traits (Matlack 1992). To this point, seed mass has been found to increase with elevation for plants in the genera Solidago (Hirano et al. 2017), and more broadly in plants that span broad elevational gradients in Australia (Satyanti et al. 2018). In addition, invasive plants richness usually increases with the increased disturbance intensity of anthropogenic disturbances in different kinds of habitats (Alston and Richardson 2006; Pinke et al. 2011). It is probably because the seed dispersal and seedling recruitment of invasive plants usually can be promoted by heavy traffic and soil disturbance (Kröel-Dulay et al. 2018; Lemke et al. 2019). Therefore, the trends we found in our field survey could instead be indicative of successful adaptation to montane environments and different kind of disturbance, which may imply greater invasion success.
We speculate that our results are more supportive of the first hypothesis, particularly because a trade-off between dispersal ability and seed mass has broad support in the literature (Cappuccino et al. 2002; Huang et al. 2015; Tabassum and Leishman 2017; DiTommaso et al. 2018). Although the NSC increased with altitude (Fig. 2B), the number of capitulum and total seed production per plant decreased (Liu et al. 2016b) suggesting that overall fecundity is reduced at high elevation. However, a more detailed study on the relationship between the traits we studied and dispersal success at our study site is required before making further conclusions.
Population genetic differentiation
We found strong support that the observed interpopulation trait variation across elevation was driven by genetic variation rather than by phenotypic plasticity. We also found that the genetic diversity of populations decreased with increasing elevation (Fig. 3). Together, our results suggest that genetic differentiation occurs during the expansion of G. quadriradiata along elevation and that the resulting genetic variation results in more homogeneous communities. These results agree with previous studies of population-level genetic characteristics, which have demonstrated that marginal populations exhibit lower genetic diversity and higher genetic differentiation than central populations (Lammi et al. 1999; Eckert et al. 2008; Excoffier and Ray 2008). Theoretically, this is expected to occur when two key genetic parameters, effective population size (Ne) and the rate of gene flow (m), are strongly influenced by the demography and spatial distribution of populations, with optimal parameter values in central populations and less optimal values in marginal populations (Excoffier and Ray 2008). Our results agree with the theoretical predictions of genetic differentiation and intrapopulation diversity, so we conclude that similar dynamics are likely to have occurred for our study species.
The phylogenetic tree showed that the smallest group is made up of different populations from the same mountain (Fig. 4). However, as the number of branches decreased, the result of clustering became chaotic. Populations from the Qinling Mountains were clustered with others from the Bashan Mountains, suggesting that there are high rates of gene flow among the populations of the two large mountains. It seems unlikely that gene flow is occurring naturally between the two mountain ranges given the distances involved, so we instead speculate that these similarities could be the result of human activities. This region of China has recently experienced increased development with a greater number of roads and increasing traffic (Liu et al. 2016b; Smith et al. 2020). The additional disturbance caused by the increased construction and number of visitors (Alston and Richardson 2006; Pauchard et al. 2009), as well as the additional dispersal route created by people who may incidentally disperse seeds between the two mountains (Liu et al. 2016b), could both lead to the phylogenetic results that we observed in this study.
Mechanisms for changing dispersal traits
Abiotic filtering can inhibit the invasion process of invasive species in mountain ranges because of the time that is typically necessary for genetic adaptation (Marini et al. 2012). Plasticity acts at the level of the individual and thus can enable organisms to adapt and survive in rapidly changing environments (Fox et al. 2019), potentially accelerating the rate which populations can adapt to abiotic filtering. Most invasive species found at high altitudes are suggested to have high adaptation ability associated with strong phenotypic adjustment capabilities (Haider et al. 2010; Alexander and Levine 2019). This phenotypic adjustment capability may involve both phenotypic plasticity and genetically based trait differentiation (Ghalambor et al. 2007; Hoffmann and Sgro 2011; Halbritter et al. 2018). For example, many invasive plants are believed to be experience high phenotypic plasticity at the beginning of an invasion, while adaptation lags behind (van Kleunen et al. 2010; Molina-Montenegro et al. 2016; Hiatt and Flory 2020).
In our field survey, we observed that the MAR of G. quadriradiata seeds increased with elevation (Fig. 2F), possibly due to a combination of phenotypic plasticity and genetic differentiation. However, we also found that MAR decreased with elevation in the greenhouse study (Fig. 5D), when seeds from different populations were grown in a shared, non-limiting environment. Mass–area ratio first increased and then decreased with elevation in the field, but it consistently decreased with source elevation in the greenhouse study [seeSupporting Information—Fig. S1], suggesting that dispersal-related traits are in part determined by plastic responses to environmental conditions. Therefore, we speculate that phenotypic plasticity also plays a considerable role in the invasion dynamics of this species. This is further evidenced by our findings that the MAR of the greenhouse populations was much lower than that in the field [seeSupporting Information—Fig. S1], MAR of the highest population decreased by 94.10 % while that of the lowest population decreased by 88.50 %, relative to in the field.
This inconsistent variation along elevational gradients between dispersal architecture (especially pappus length) and other dispersal-related traits has also been detected by others (Gravuer et al. 2003). It may be caused by environmental variation in mountain ranges that could underlie the effects of inherent genetic differentiation. Environmental variation was suppressed in the greenhouse, thus decreasing phenotypic variation of dispersal-related traits. Along the elevational gradient, the changing environmental conditions caused dispersal-related traits to change in ways that did not reflect the interpopulation genetic differentiation. Therefore, our results indicate that phenotypic plasticity also plays an important role in the expansion of G. quadriradiata in mountain ranges. Furthermore, it indicates that mountains could play a key role in slowing down the dispersion of G. quadriradiata not only directly by abiotic factors but also indirectly by changing its dispersal-related traits. Therefore, we conclude that variation of dispersal-related traits for this species reflects genetic divergence from low elevation conditions. We further speculate that the trait variation reflects changes in dispersal ability; however, further research will be needed to ascertain this. Regardless, this study provides empirical evidence for the evolution of dispersal-related traits in the process of plant invasion into high mountain ranges.
Conclusions
Trait variation among populations has been associated with changes in natural selection pressure along elevation (Bode and Dufresne 2019). Long-term adaptation to environmental factors would thus result in genetically based changes in phenotypic traits (Marin et al. 2019; Pimpinelli et al. 2019). In this study, patterns of genetic differentiation were found to be associated with elevational differences among populations of G. quadriradiata in central China. This differentiation was also associated with dispersal-related traits of this species, suggesting that dispersal ability may be genetically based. Moreover, differentiation in MAR between elevational populations in the greenhouse experiment (Fig. 5D; seeSupporting Information—Fig. S1) suggests that trait variation may also be partially controlled by phenotypic plasticity, echoing results from other studies (Holtsford and Ellstrand 1992). Our results therefore indicate that genetically based rapid evolution, as well as phenotypic plasticity, play important role in the expansion of G. quadriradiata in mountain ranges. Although our results support the idea that mountain ranges can act as natural barriers to plant invasions, the plasticity demonstrated in the greenhouse experiment implies that once acclimated to high elevations, acclimating back to low elevations on the other side will occur quickly and therefore should be cautiously monitored. Further research is needed to link the traits from this study directly to dispersal ability as well as to investigate these patterns across broader geographic gradients.
Supporting Information
The following additional information is available in the online version of this article—
Figure S1. MAR (mass–area ratio) of the four populations in greenhouse experiment and field.
Table S1. The random effects of linear mixed-effect models on MAR (mass–area ratio), HSW (weight of one hundred seeds), NSC (Number of mature seed per capitulum), seed length, pappus length and pappus width.
Table S2. The random effects of linear mixed-effect models on PPL, I and He.
Acknowledgements
We thank Chuan-Fei Li, Xiao Chang, Lan-Yue Li, Qing-Qing Chen, Xiong Shi, Xin-Wen Cai, Zhuo-Lu Ren, Na-Na Yao, Wen-Tao Peng and Hui-Li Zhu for assisting in field survey and seed collection. We thank Dr Daijiang Li for the comments on the manuscript.
Populations & Communities. Chief Editor: Jean Burns
Sources of Funding
This work was supported by the National Natural Science Foundation of China (31600445, 31570425 and 11671243), the Natural Science Basic Research Plan in Shaanxi Province of China (2020JM-286), the Fundamental Research Funds for the Central Universities (GK202103072, 2019CSLY019 and 2020CSLY014).
Conflict of Interest
The authors declare no competing of interests.
Data Availability
The data used in this study are available as Supporting Information.
Literature Cited
- Alexander JM, Chalmandrier L, Lenoir J, Burgess TI, Essl F, Haider S, Kueffer C, McDougall K, Milbau A, Nuñez MA, Pauchard A, Rabitsch W, Rew LJ, Sanders NJ, Pellissier L. 2018. Lags in the response of mountain plant communities to climate change. Global Change Biology 24:563–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JM, Lembrechts JJ, Cavieres LA, Daehler C, Haider S, Kueffer C, Liu G, McDougall K, Milbau A, Pauchard A, Rew LJ, Seipel T. 2016. Plant invasions into mountains and alpine ecosystems: current status and future challenges. Alpine Botany 126:89–103. [Google Scholar]
- Alexander JM, Levine JM. 2019. Earlier phenology of a nonnative plant increases impacts on native competitors. Proceedings of the National Academy of Sciences of the United States of America 116:6199–6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JM, Poll M, Dietz H, Edwards PJ. 2009. Contrasting patterns of genetic variation and structure in plant invasions of mountains. Diversity and Distributions 15:502–512. [Google Scholar]
- Alford RA, Brown GP, Schwarzkopf L, Phillips BL, Shine R. 2009. Comparisons through time and space suggest rapid evolution of dispersal behaviour in an invasive species. Wildlife Research 36:23–28. [Google Scholar]
- Alston KP, Richardson DM. 2006. The roles of habitat features, disturbance, and distance from putative source populations in structuring alien plant invasions at the urban/wildland interface on the Cape Peninsula, South Africa. Biological Conservation 132:183–198. [Google Scholar]
- Alzate A, Onstein RE, Etienne RS, Bonte D. 2020. The role of preadaptation, propagule pressure and competition in the colonization of new habitats. Oikos 129:820–829. [Google Scholar]
- Andrade-Restrepo M, Champagnat N, Ferrière R. 2019. Local adaptation, dispersal evolution, and the spatial eco-evolutionary dynamics of invasion. Ecology Letters 22:767–777. [DOI] [PubMed] [Google Scholar]
- Augspurger CK, Franson SE. 1987. Wind dispersal of artifical fruits varying in mass, area, and morphology. Ecology 68:27–42. [Google Scholar]
- Becker T, Dietz H, Billeter R, Buschmann H, Edwards PJ. 2005. Altitudinal distribution of alien plant species in the Swiss Alps. Perspectives in Plant Ecology, Evolution and Systematics 7:173–183. [Google Scholar]
- Bode RF, Dufresne C. 2019. Natural selection on flower size in invasive Cytisus scoparius along an elevation gradient. Journal of Plant Ecology 13:165–170. [Google Scholar]
- Bustamante RO, Durán AP, Peña-Gómez FT, Véliz D. 2018. Genetic and phenotypic variation, dispersal limitation and reproductive success in the invasive herb Eschscholzia californica along an elevation gradient in central Chile. Plant Ecology & Diversity 10:419–429. [Google Scholar]
- Cappuccino N, Mackay R, Eisner C. 2002. Spread of the invasive alien vine Vincetoxicum rossicum: tradeoffs between seed dispersability and seed quality. The American Midland Naturalist 148:263–270. [Google Scholar]
- Carboni M, Guéguen M, Barros C, Georges D, Boulangeat I, Douzet R, Dullinger S, Klonner G, van Kleunen M, Essl F, Bossdorf O, Haeuser E, Talluto MV, Moser D, Block S, Conti L, Dullinger I, Münkemüller T, Thuiller W. 2018. Simulating plant invasion dynamics in mountain ecosystems under global change scenarios. Global Change Biology 24:e289–e302. [DOI] [PubMed] [Google Scholar]
- Davidson AM, Jennions M, Nicotra AB. 2011. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecology Letters 14:419–431. [DOI] [PubMed] [Google Scholar]
- DiTommaso A, Stokes CA, Cordeau S, Milbrath LR, Whitlow TH. 2018. Seed-dispersal ability of the invasive perennial vines Vincetoxicum nigrum and Vincetoxicum rossicum. Invasive Plant Science and Management 11:10–19. [Google Scholar]
- Drollinger S, Müller M, Kobl T, Schwab N, Böhner J, Schickhoff U, Scholten T. 2017. Decreasing nutrient concentrations in soils and trees with increasing elevation across a treeline ecotone in Rolwaling Himal, Nepal. Journal of Mountain Science 14:843–858. [Google Scholar]
- Dytham C. 2009. Evolved dispersal strategies at range margins. Proceedings of the Royal Society of London, B. Biological Sciences 276:1407–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert CG, Samis KE, Lougheed SC. 2008. Genetic variation across species’ geographical ranges: the central-marginal hypothesis and beyond. Molecular Ecology 17:1170–1188. [DOI] [PubMed] [Google Scholar]
- Ensslin A, Fischer M. 2015. Variation in life-history traits and their plasticities to elevational transplantation among seed families suggests potential for adaptative evolution of 15 tropical plant species to climate change. American Journal of Botany 102:1371–1379. [DOI] [PubMed] [Google Scholar]
- Estrada A, Morales-Castilla I, Caplat P, Early R. 2016. Usefulness of species traits in predicting range shifts. Trends in Ecology & Evolution 31:190–203. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Ray N. 2008. Surfing during population expansions promotes genetic revolutions and structuration. Trends in Ecology & Evolution 23:347–351. [DOI] [PubMed] [Google Scholar]
- Fox RJ, Donelson JM, Schunter C, Ravasi T, Gaitán-Espitia JD. 2019. Beyond buying time: the role of plasticity in phenotypic adaptation to rapid environmental change. Philosophical Transactions of the Royal Society of London, Series B. Biological Sciences 374:20180174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei ER, Ghazoul J, Matter P, Heggli M, Pluess AR. 2014. Plant population differentiation and climate change: responses of grassland species along an elevational gradient. Global Change Biology 20:441–455. [DOI] [PubMed] [Google Scholar]
- Gawel N, Jarret R. 1991. A modified CTAB DNA extraction procedure for Musa and Ipomoea. Plant Molecular Biology Reporter 9:262–266. [Google Scholar]
- Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology 21:394–407. [Google Scholar]
- Gravuer K, von Wettberg EJ, Schmitt J. 2003. Dispersal biology of Liatris scariosa var. novae-angliae (Asteraceae), a rare New England grassland perennial. American Journal of Botany 90:1159–1167. [DOI] [PubMed] [Google Scholar]
- Haider S, Alexander J, Dietz H, Trepl L, Edwards PJ, Kueffer C. 2010. The role of bioclimatic origin, residence time and habitat context in shaping non-native plant distributions along an altitudinal gradient. Biological Invasions 12:4003–4018. [Google Scholar]
- Halbritter AH, Fior S, Keller I, Billeter R, Edwards PJ, Holderegger R, Karrenberg S, Pluess AR, Widmer A, Alexander JM. 2018. Trait differentiation and adaptation of plants along elevation gradients. Journal of Evolutionary Biology 31:784–800. [DOI] [PubMed] [Google Scholar]
- Hargreaves AL, Eckert CG, Bailey J. 2014. Evolution of dispersal and mating systems along geographic gradients: implications for shifting ranges. Functional Ecology 28:5–21. [Google Scholar]
- Hiatt D, Flory SL. 2020. Populations of a widespread invader and co-occurring native species vary in phenotypic plasticity. The New Phytologist 225:584–594. [DOI] [PubMed] [Google Scholar]
- Hirano M, Sakaguchi S, Takahashi K. 2017. Phenotypic differentiation of the Solidago virgaurea complex along an elevational gradient: insights from a common garden experiment and population genetics. Ecology and Evolution 7:6949–6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Sgrò CM. 2011. Climate change and evolutionary adaptation. Nature 470:479–485. [DOI] [PubMed] [Google Scholar]
- Holtsford TP, Ellstrand NC. 1992. genetic and environmental variation in floral traits affecting outcrossing rate in Clarkia tembloriensis (Onagraceae). Evolution 46:216–225. [DOI] [PubMed] [Google Scholar]
- Huang FF, Peng SL, Chen BM, Liao HX, Huang QQ, Lin ZG, Liu G. 2015. Rapid evolution of dispersal-related traits during range expansion of an invasive vine Mikania micrantha. Oikos 124:1023–1030. [Google Scholar]
- Kabuce N, Priede N. 2010. Nobanis - invasive alien species fact sheet - Galinsoga quadriradiata. From: Online database of the North European and Baltic Network on invasive alien species – nobanis. www.nobanis.org (12 January 2014).
- Kanapeckas KL, Tseng TM, Vigueira CC, Ortiz A, Bridges WC, Burgos NR, Fischer AJ, Lawton-Rauh A. 2018. Contrasting patterns of variation in weedy traits and unique crop features in divergent populations of US weedy rice (Oryza sativa sp.) in Arkansas and California. Pest Management Science 74:1404–1415. [DOI] [PubMed] [Google Scholar]
- Körner C. 2007. The use of ‘altitude’ in ecological research. Trends in Ecology & Evolution 22:569–574. [DOI] [PubMed] [Google Scholar]
- Kröel-Dulay G, Csecserits A, Szitár K, Molnár E, Szabó R, Ónodi G, Botta-Dukát Z. 2018. The potential of common ragweed for further spread: invasibility of different habitats and the role of disturbances and propagule pressure. Biological Invasions 21:137–149. [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution 35:1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeseke P, Martínez B, Mansilla A, Bischof K. 2020. Future range dynamics of the red alga Capreolia implexa in native and invaded regions: contrasting predictions from species distribution models versus physiological knowledge. Biological Invasions 22:1339–1352. [Google Scholar]
- LaForgia ML, Harrison SP, Latimer AM. 2020. Invasive species interact with climatic variability to reduce success of natives. Ecology 101:e03022. [DOI] [PubMed] [Google Scholar]
- Lammi A, Siikamäki P, Mustajärvi K. 1999. Genetic diversity, population size, and fitness in central and peripheral populations of a rare plant Lychnis viscaria. Conservation Biology 13:1069–1078. [Google Scholar]
- Lemke A, Kowarik I, von der Lippe M. 2019. How traffic facilitates population expansion of invasive species along roads: the case of common ragweed in Germany. Journal of Applied Ecology 56:413–422. [Google Scholar]
- Li z, Dong Y, Wu S, Huang H. 1990. The climatological characteristics of the Bashan Mountains. Acta Geographica Sinica 45:311–320. [Google Scholar]
- Liao HX, D’Antonio CM, Chen BM, Huang QQ, Peng SL. 2016. How much do phenotypic plasticity and local genetic variation contribute to phenotypic divergences along environmental gradients in widespread invasive plants? A meta-analysis. Oikos 125:905–917. [Google Scholar]
- Liu G, Gao Y, Huang FF, Yuan MY, Peng SL. 2016a. The invasion of coastal areas in South China by Ipomoea cairica may be accelerated by the ecotype being more locally adapted to salt stress. PLoS One 11:e0149262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yang YB, Zhu ZH. 2018. Elevated nitrogen allows the weak invasive plant Galinsoga quadriradiata to become more vigorous with respect to inter-specific competition. Scientific Reports 8:3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zhang LL, Kong BB, Wei XH, Zhu ZH. 2016b. The population growth dynamic of Galinsoga quadriradiata Ruiz & Pav. on Qinling-Bashan Mountain. Acta Ecologica Sinica 36:3350–3361. [Google Scholar]
- Marin P, Genitoni J, Barloy D, Maury S, Gibert P, Ghalambor CK, Vieira C, Herrel A. 2019. Biological invasion: the influence of the hidden side of the (epi)genome. Functional Ecology 34:385–400. [Google Scholar]
- Marini L, Battisti A, Bona E, Federici G, Martini F, Pautasso M, Hulme PE. 2012. Alien and native plant life-forms respond differently to human and climate pressures. Global Ecology and Biogeography 21:534–544. [Google Scholar]
- Matlack GR. 1987. Diaspore size, shape, and fall behavior in wind-dispersed plant species. American Journal of Botany 74:1150–1160. [Google Scholar]
- Matlack GR. 1992. Influence of fruit size and weight on wind dispersal in Betula lenta, a gap-colonizing tree species. The American Midland Naturalist 128:30–39. [Google Scholar]
- Meyer S, Carlson S. 2001. Achene mass variation in Ericameria nauseosus (Asteraceae) in relation to dispersal ability and seedling fitness. Functional Ecology 15:274–281. [Google Scholar]
- Molina-Montenegro MA, del Pozo A, Gianoli E. 2016. Ecophysiological basis of the Jack-and-Master strategy: Taraxacum officinale (dandelion) as an example of a successful invader. Journal of Plant Ecology 11:147–157. [Google Scholar]
- Monty A, Mahy G. 2010. Evolution of dispersal traits along an invasion route in the wind-dispersed Senecio inaequidens (Asteraceae). Oikos 119:1563–1570. [Google Scholar]
- Monty A, Stainier C, Lebeau F, Pieret N, Mahy G. 2008. Seed rain pattern of the invasive weed Senecio inaequidens (Asteraceae). Belgian Journal of Botany 141:51–63. [Google Scholar]
- Nei M. 1987. Molecular evolutionary genetics. New York, NY: Columbia University Press. [Google Scholar]
- Pauchard A, Kueffer C, Dietz H, Daehler C, Alexander J, Edwards P, Arévalo JR, Cavieres L, Guisan A, Haider S, Jakobs G, McDougall K, Millar C, Naylor B, Parks C, Rew L, Seipel T. 2009. Ain’t no mountain high enough: plant invasions reaching new elevations. Frontiers in Ecology and the Environment 7:479–486. [Google Scholar]
- Peakall R, Smouse PE. 2012. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research–an update. Bioinformatics 28:2537–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips BL, Brown GP, Shine R. 2010. Life-history evolution in range-shifting populations. Ecology 91:1617–1627. [DOI] [PubMed] [Google Scholar]
- Pimpinelli S, Piacentini L, Herrel A. 2019. Environmental change and the evolution of genomes: transposable elements as translators of phenotypic plasticity into genotypic variability. Functional Ecology 34:428–441. [Google Scholar]
- Pinke G, Karácsony P, Czúcz B, Botta-Dukát Z. 2011. Environmental and land-use variables determining the abundance of Ambrosia artemisiifolia in arable fields in Hungary. Preslia 83:219–235. [Google Scholar]
- Qian W, Ge S. 2001. Analyses of population genetic structure by using dominant markers. Acta Genetica Sinica 28:244–255. [PubMed] [Google Scholar]
- R Development Core Team. 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rejmanek M, Richardson DM. 1996. What attributes make some plant species more invasive? Ecology 77:1655–1661. [Google Scholar]
- Rejmánek M, Richardson DM, Pyšek P. 2013. Plant invasions and invasibility of plant communities. In: van der Maarel E, Franklin J, eds. Vegetation ecology, 2nd edn. Hoboken, NJ: John Wiley and Sons, 387–424.
- Satyanti A, Nicotra AB, Merkling T, Guja LK. 2018. Seed mass and elevation explain variation in seed longevity of Australian alpine species. Seed Science Research 28:319–331. [Google Scholar]
- Seale M, Nakayama N. 2020. From passive to informed: mechanical mechanisms of seed dispersal. The New Phytologist 225:653–658. [DOI] [PubMed] [Google Scholar]
- Shine R, Brown GP, Phillips BL. 2011. An evolutionary process that assembles phenotypes through space rather than through time. Proceedings of the National Academy of Sciences of the United States of America 108:5708–5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL, Hodkinson TR, Villellas J, Catford JA, Csergő AM, Blomberg SP, Crone EE, Ehrlén J, Garcia MB, Laine AL, Roach DA, Salguero-Gómez R, Wardle GM, Childs DZ, Elderd BD, Finn A, Munné-Bosch S, Baudraz MEA, Bódis J, Brearley FQ, Bucharova A, Caruso CM, Duncan RP, Dwyer JM, Gooden B, Groenteman R, Hamre LN, Helm A, Kelly R, Laanisto L, Lonati M, Moore JL, Morales M, Olsen SL, Pärtel M, Petry WK, Ramula S, Rasmussen PU, Enri SR, Roeder A, Roscher C, Saastamoinen M, Tack AJM, Töpper JP, Vose GE, Wandrag EM, Wingler A, Buckley YM. 2020. Global gene flow releases invasive plants from environmental constraints on genetic diversity. Proceedings of the National Academy of Sciences of the United States of America 117:4218–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soethe N, Lehmann J, Engels C. 2008. Nutrient availability at different altitudes in a tropical montane forest in Ecuador. Journal of Tropical Ecology 24:397–406. [Google Scholar]
- Steyn C, Greve M, Robertson MP, Kalwij JM, Roux PCl. 2017. Alien plant species that invade high elevations are generalists_ support for the directional ecological filtering hypothesis. Journal of Vegetation Science 28:337–346. [Google Scholar]
- Tabassum S, Leishman MR. 2017. Have your cake and eat it too: greater dispersal ability and faster germination towards range edges of an invasive plant species in eastern Australia. Biological Invasions 20:1199–1210. [Google Scholar]
- Thomson FJ, Letten AD, Tamme R, Edwards W, Moles AT. 2018. Can dispersal investment explain why tall plant species achieve longer dispersal distances than short plant species? The New Phytologist 217:407–415. [DOI] [PubMed] [Google Scholar]
- Travis JM, Dytham C. 2002. Dispersal evolution during invasions. Evolutionary Ecology Research 4:1119–1129. [Google Scholar]
- Ueno S, Rodrigues JF, Alves-Pereira A, Pansarin ER, Veasey EA. 2015. Genetic variability within and among populations of an invasive, exotic orchid. AoB PLANTS 7:plv077; doi: 10.1093/aobpla/plv077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kleunen M, Weber E, Fischer M. 2010. A meta-analysis of trait differences between invasive and non-invasive plant species. Ecology Letters 13:235–245. [DOI] [PubMed] [Google Scholar]
- Vitasse Y, Lenz A, Kollas C, Randin CF, Hoch G, Körner C, Whitehead D. 2014. Genetic vs. non-genetic responses of leaf morphology and growth to elevation in temperate tree species. Functional Ecology 28:243–252. [Google Scholar]
- Volis S, Ormanbekova D, Yermekbayev K. 2015. Role of phenotypic plasticity and population differentiation in adaptation to novel environmental conditions. Ecology and Evolution 5:3818–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Zhang P, Liang L, Zhang S. 2017. Evaluation of precipitation from CMORPH, GPCP-2, TRMM 3B43, GPCC, and ITPCAS with ground-based measurements in the Qinling-Daba Mountains, China. PLoS One 12:e0185147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watermann LY, Hock M, Blake C, Erfmeier A. 2019. Plant invasion into high elevations implies adaptation to high UV-B environments: a multi-species experiment. Biological Invasions 22:1203–1218. [Google Scholar]
- Williams JL, Hufbauer RA, Miller TEX. 2019. How evolution modifies the variability of range expansion. Trends in Ecology & Evolution 34:903–913. [DOI] [PubMed] [Google Scholar]
- Yang YB, Liu G, Shi X, Zhang WG, Cai XW, Ren ZL, Yao NN, Zhu ZH, Nie H. 2018. Where will invasive plants colonize in response to climate change: predicting the invasion of Galinsoga quadriradiata in China. International Journal of Environmental Research 12:929–938. [Google Scholar]
- Zhan A, Li C, Fu J. 2009. Big mountains but small barriers: population genetic structure of the Chinese wood frog (Rana chensinensis) in the Tsinling and Daba Mountain region of northern China. BMC Genetics 10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XG, Ma CH, Xiao L. 2014. The vegetation history of Qinling Mountains, China. Quaternary International 325:55–62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this study are available as Supporting Information.