Figure 1.
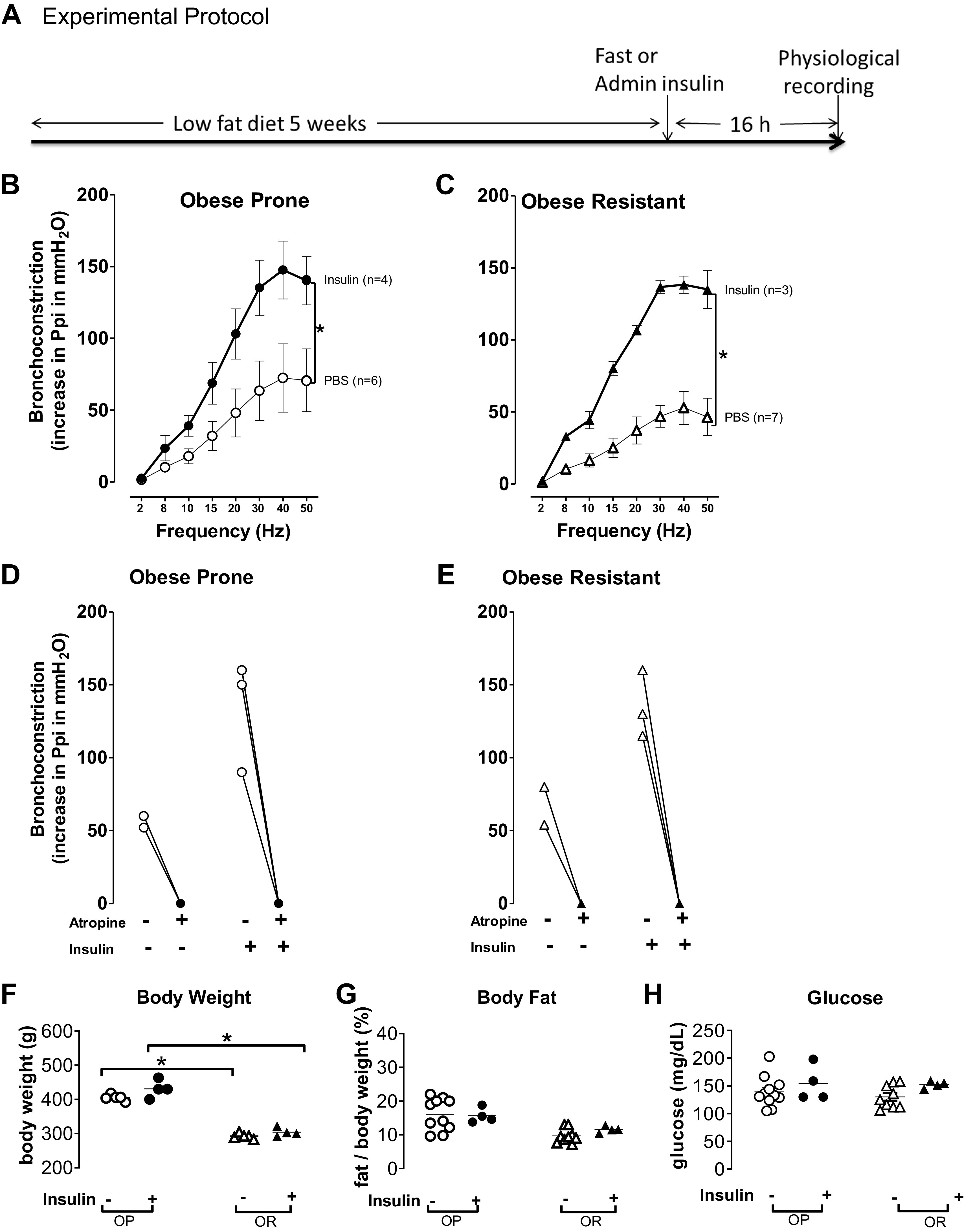
Insulin-potentiated vagally induced bronchoconstriction. A: obese-prone (OP) and obese-resistant (OR) rats were fed a low-fat diet for 5 wk. Sixteen hours before measuring airway function, rats were either treated with 100 μL of phosphate-buffered saline (PBS) subcutaneously and fasted overnight or treated with supplemental insulin (Lantus, 3 units/rat subcutaneously) with free access to food. B–C: electrical stimulation of both vagus nerves (2–50 Hz, 20 V, 0.4 ms pulse duration, for 6 s at 40 s intervals) caused frequency-dependent bronchoconstriction that was not significantly different between obese-prone rats (B, open circles) and obese-resistant rats (C, open triangles) treated with PBS. After a subcutaneous injection of insulin 16 h before measuring airway physiology, insulin significantly potentiated bronchoconstriction in both obese-prone (B, filled circles) and obese-resistant rats (C, filled triangles). D and E: atropine blocked vagally induced bronchoconstriction at 50 Hz (open symbols, bronchoconstriction before atropine; filled symbols, bronchoconstriction after atropine). F: obese-prone rats (OP, circles) on a high-fat diet for 5 wk weighed significantly more than obese-resistant (OR, triangles) rats on the same diet, regardless of insulin treatment. G: body fat, as a % of weight, was not significantly different among any groups. H: in rats not treated with insulin, fasting glucose was not different between obese-prone (open circles) or obese-resistant (open triangles) rats. Glucose was also not different between obese-prone and -resistant rats treated with insulin and with unlimited access to food, (filled symbols). Data are represented as means ± SE. *P < 0.05.
