Abstract
Background:
Glioblastoma multiforme (GBM) owes an ominous prognosis: its mean overall survival is 14 months. The extent of surgical resection (ESR) highlights among factors in which an association has been found to a somewhat better prognosis. However, the association between greater ESR and prolonged overall (OS) survival is not a constant finding nor a proven cause-and-effect phenomenon. To our objective is to establish the strength of association between ESR and OS in patients with GBM through a systematic review and meta-analysis.
Methods:
In accordance with PRISMA-P recommendations, we conducted a systematic literature search; we included studies with adult patients who had undergone craniotomy for GBM. Our primary outcome is overall postoperative survival at 12 and 24 months. We reviewed 180 studies, excluded 158, and eliminated 8; 14 studies that suited our requirements were analyzed.
Results:
The initial level of evidence of all studies is low, and it may be degraded to very low according to GRADE criteria because of design issues. The definition of different levels of the extent of resection is heterogeneous and poorly defined. We found a great amount of variation in the methodology of the operation and the adjuvant treatment protocol. The combined result for relative risk (RR) for OS for 12 months analysis is 1.25 [95% confidence interval (95% CI) 1.14–1.36, P < .01], absolute risk reduction (ARR) of 15.7% (95% CI 11.9–19.4), relative risk reduction (RRR) of 0.24 (95% CI 0.18–0.31), number needed to treat (NNT) 6; for 24-month analysis RR is 1.59 (95% CI 1.11–2.26, P < .01) ARR of 11.5% (95% CI 7.7–15.1), relative risk reduction (RRR) of 0.53 (95% CI 0.33–0.76), (NNT) 9. In each term analysis, the proportion of alive patients who underwent more extensive resection is significantly higher than those who underwent subtotal resection.
Conclusion:
Our results sustain a weak but statistically significant association between the ESR and OS in patients with GBM obtained from observational studies with a very low level of evidence according to GRADE criteria. As a consequence, any estimate of effect is very uncertain. Current information cannot sustain a cause-and-effect relationship between these variables.
Keywords: extent of resection, glioblastoma multiforme, prognosis, surgical resection, survival
1. Introduction
Malignancies in the brain account for approximately 2% of the oncological disease in humans[1]; glioblastoma multiforme (GBM) is the most common malignant brain tumor in adults and is well known for its aggressive behavior and its invasiveness.[2–4] Despite its low incidence in the general population, it constitutes a multifaceted problem: its diagnosis is always a tragic event for the patient and their family and a challenging situation to manage for health care systems around the world. Even with the best current multidisciplinary treatment, the average survival after diagnosis is only 14 months.[5]
Despite the high level of research, in the last 2 decades, there has not been a significant improvement in the ominous prognosis of patients with GBM.[4–7] The standard of treatment for a patient with magnetic resonance imaging (MRI) suggestive of a high-grade glioma is multidisciplinary evaluation and surgical intervention aimed at maximum safe resection, followed by radiation therapy and concomitant adjuvant chemotherapy.[8]
One subject of extensive surgical research is the influence of the extent of surgical tumor resection (ESR) on the prognosis of survival. Surgical intervention is considered the cornerstone of treatment: it obtains and provides tissue for histopathological, genetic, and molecular analysis, can improve the initial neurological status through proper resection and decompression, and can contribute to subsequent adjuvant treatment through cytoreductive surgery. Current literature reports that overall survival ranges from 52 to 86 weeks when surgical resection is larger than 98% and from 35 to 64 weeks if the resection is less.[3,9–13] However, achieving “total” resection finds many challenges[14,15]: First, GBM is a highly infiltrative tumor; as noted by studies of specimens obtained by stereotactic biopsy and in vitro cell culture, brain areas peripheral to tumor areas visible in contrast-enhanced MRI are usually infiltrated by tumor cells at the time of diagnosis[16,17]; Second, the best possibility of determining the preoperative extent is magnetic resonance imaging; however, there is debate over which acquisition most adequately shows the size of the tumor[18,19]; third, the neoplasia visible on MRI can, from the moment of diagnosis, invade eloquent or vital brain areas, making complete resection impossible. Fourth, any benefit obtained from more extensive resection is nullified if the resection produces a greater neurological deficit than the preoperative deficit because alterations in the neurological condition of patients are associated with a decrease in overall survival.[19–21]
In surgery, the similarity in appearance of the tumor with the surrounding brain has posed a challenge for surgeons[22,23] but has recently improved with the use of neuronavigation, intraoperative magnetic resonance and ultrasound, and fluorescein-guided technology,[15] aiming for maximum possible resection; however, the rationale behind this objective is not yet defined. The concept is mainly based on retrospective studies, and it is still debatable.[15] There is no consensus on the extent of optimal resection to improve survival,[2,19] and multiple articles conclude that they do not find an association between the extent of resection and improved survival rates,[24–26] a concept that has been expressed in clinically based research work, like the one from Poon et al,[27] that sustains that “multiple clinical trials had investigated novel therapies that showed promise in pre-clinical and early phase studies, but to date, there have been no significant additions to the treatment armamentarium for newly diagnosed patients since 2005”… when “a landmark clinical trial … demonstrated that the addition of concomitant and adjuvant temozolomide to radiotherapy provided an additional survival benefit to patients diagnosed with glioblastoma.”[2]
The concept of ”maximum safe resection“ corresponds more to a philosophical concept than to a defined surgical objective: there is no standard that evaluates surgical planning or the surgical result. In cohort studies that have assessed the medical care of GBM, there is wide variation in the performance of surgical procedures,[28] that is, surgical intervention, the cornerstone of oncological treatment of GBM, can range from minimally invasive biopsy to a craniotomy with ”total“ resection.[2,19,29] Until recently, the extent of surgical resection was not based on imaging studies but on the judgment of the surgeon, who systematically tend to overestimate it.[30,31]
We have not found a current publication with any protocol that consistently demonstrates improvement in overall survival (not recurrence-free survival) of patients with GBM based on the ESR. Still, the debate is an ongoing issue[4–6] going beyond the proportion of tumor resection and extending to other relevant parallel issues: measuring resection given that GBM is highly invasive neoplasia, the influence of tumor invasion, or surgical manipulation on some anatomical regions, such as the caudate nucleus, thalamus, and ventricles, and even the influence of tumor shape on the prognosis of these patients.[28,32]
As some authors have suggested, the ESR may be a surrogate parameter for other variables. Nevertheless, it is frequently associated with the primary objective of oncological treatment: more prolonged overall survival.[3,33,34]
2. Objective
To evaluate in current literature the impact that the ESR may have on the overall survival of patients with GBM, specifically, whether it is currently possible to conclude that different extents of tumor resection produce differences in survival times.
The primary outcome is the number of patients who survived at 12 and 24 months after maximum or sub-maximum surgical resection of the tumor.
3. Materials and methods
The protocol was carried out according to PRISMA-P recommendations.[35]
The Protocol was presented to the Anahuac University Research and Ethics Committee, it was accepted for its development on November 2019, with acceptance number 1371919.
3.1. Systematic review
We conducted a systematic literature search querying the following databases: CENTRAL, The Cochrane Library, Embase, Google Scholar, Ovid, LILACS y Pubmed. Search Strategy was based on the following medical subject headings (MeSH) and keywords
”glioblastoma,“ glioblastoma multiforme,” “extent of resection,” “resection,” “overall survival,” and “progression-free survival.”
We restricted the search results to documents in English and Spanish languages and to publications made between January 1, 2000, and July 31, 2019. With the purpose of extending our search, the function “related articles” of the search engines was used; we also examined the references of selected articles to enhance de review.
The inclusion criteria are studies from 2000 to 2019, which report patients with a new diagnosis of GBM, in which the relationship between the extent of surgical resection and overall survival reported at 12 and 24 months is reported and measured in the same level of measurement.
For our study, the operational definition of the independent variable ESR is the amount of tumor (GBM) volume, percentage, or ratio that was resected during a surgical intervention.
For our study, the measurement of the primary outcome variable---survival at 12 and 24 months---is a nominal dichotomic one.
A total of 180 studies were found; its titles and abstracts were carefully analyzed independently by 2 of the researchers and defined as appropriate to be included or excluded. The software Covidence (VERITAS HEALTH INNOVATION. Covidence systematic review software. 2017https://www.covidence.org/home) was used to aid in managing the selection process.
Studies comparing the overall survival of patients with GBM with different percentages of tumor resection were included.
The exclusion criteria included studies that included tumor diagnosis different to GBM, studies with multiple tumor types, studies of GBM received, studies that do not report the extent of surgical resection, studies that do not report survival at 12 and 24 months, and studies that do not report the comparison of different amounts of surgical resection. Biopsy was not considered as surgical resection in this study.
Case reports, case series, studies published before the year 2000, studies that included different types of brain tumors in addition to GBM, studies that did not include the amount of surgical resection as an independent variable, studies that only investigated biopsy and not surgical resection, and studies about surgical reintervention were excluded.
Three clinical trials and 19 case--control or cohort studies fulfilled the inclusion criteria and were thoroughly reviewed[9,15,19,31,33,36–52]; all the clinical trials and 5 cohort studies were eliminated from our analysis.
One of the clinical trials[36] was eliminated from the analysis because it includes several types of brain neoplasms and because it compares biopsy with craniotomy. The second[33] was eliminated because it does not consider overall survival as an outcome variable. The third[15] was eliminated because authors, very elegantly, clarify in the text that: “The study was neither designed nor powered to show differences in long-term endpoints such as overall survival.” Four retrospective cohort studies were eliminated because dichotomized data related to overall survival could not be extracted,[9,37–39] and another was excluded because it deals with a subgroup of patients reported in a different publication.[40]
Data used to perform this meta-analysis were obtained from 14 studies that fulfilled the inclusion criteria and did not have exclusion or elimination criteria.[19,31,41–52]
PRISMA flow diagram is shown in Figure 1.
Figure 1.
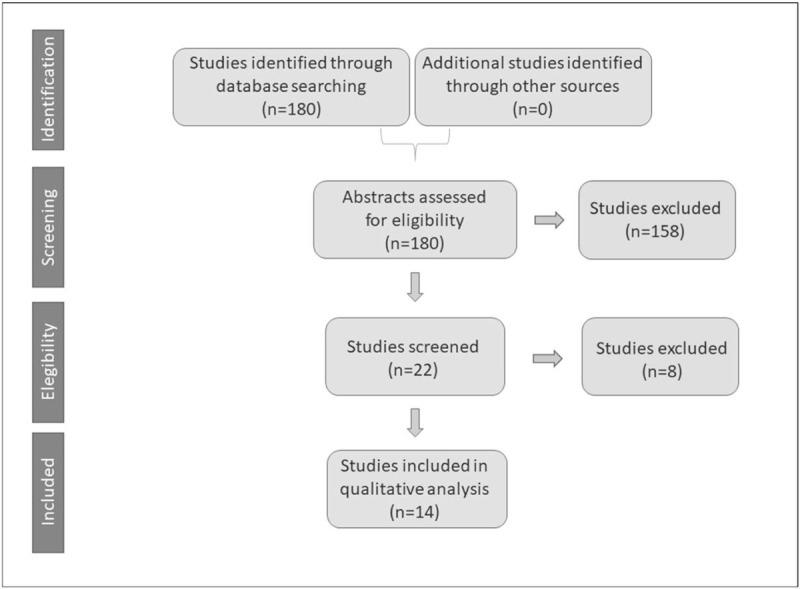
PRISMA flow diagram.
3.2. Quality and evidence grading
Quality related to the degree of evidence that studies are able to produce was evaluated according to PRISMA-P recommendations[35] independently by 2 of the investigators at study and outcome level; in case of controversy, the result was consulted and accorded with a third investigator. The reliability of data was evaluated by Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria.[53,54] The risk of bias on each study's methodology was assessed using the “Cochrane Collaboration Risk of Bias Tool”[55] and the “Newcastle-Ottawa Scale.”[56]
For evaluation of quality in each study, the design, definition, and representativeness of the patients that undergone total or more significant resections, and description and selection of patients that underwent lesser resections were noted; for evaluation of the risk of performance bias, the type of design of each study was evaluated, as well as the blinding of participants and personnel; for evaluation of selection bias, the kind of design, random sequence generation, allocation concealment, and blinding of patients were noted; for evaluation of detection bias, blinding of the evaluators was noted; for evaluation of attrition bias, loss of patients for follow-up, and loss of data regarding the outcome variable were stressed.
3.3. Data extraction
Specifically, the degree of association between the extent of resection and overall survival at 12 and 24 months was analyzed.
For comparison, the highest level of resection published in the article was used against the other levels of resection, except in the article by Blomstergren et al,[41] in which the data could not be obtained, and the second-highest level of resection was chosen. Data were manually obtained in all studies from the Kaplan--Meier survival curves published and corroborated with WebPlotDigitizer (https://automeris.io/WebPlotDigitizer) software.
In the studies that included patients with GBM who only underwent a biopsy, no comparison was made between resection and biopsy, with the intention of avoiding selection and performance bias in our analysis,[57,58] given that the reason for not attempting complete or partial tumor resection was not analyzed in these studies, including reasons might include such as the severity of the clinical or neurological status, deep tumors with extensive visible or multicentric infiltration, moribund patients, among others, but there was a clear decision on the part of the treating medical team not to carry out surgical resection, which is the primary independent variable of the analysis.
The summary measure is individual and combined relative risk.
3.4. Data analysis
Data were analyzed using IBM SPSS Statistics IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp and Excel Microsoft Corporation, 2018. Microsoft Excel, Available at: https://office.microsoft.com/excel. Association between de ESR and overall survival at 12 and 24 months was analyzed through RR computation and their 95% confidence intervals; Chi-squared test or Fisher exact test was used to determine the probability of RR being equal to 1. Alfa error was defined below 0.05. For the meta-analysis, outcomes were combined and calculated using Epidat (Version 3.1 Dirección Xeral de Saúde Pública, Consellería de Sanidade - Xunta de Galicia, Área de Análisis de Salud y Sistemas de Información Sanitaria Organization Panamericana de la Salud OPS/OMS) statistical software, according to guides of the current version of the Cochrane handbook for systematic reviews of interventions.[59]
In the case heterogeneity was not found to be statistically significant, the Mantel-Haenszel method for fixed-effects model was used.[60] If heterogeneity was found to be statistically significant (I2 ≥ 50% or P < .1), random-effects model was used.[61]
4. Results
Fourteen studies were published between 2011 and 2019, with a total of 2470 patients diagnosed with GBM, were included. In the studies in which biopsy patients were included, these were subtracted from the analysis; patients in whom the only surgical procedure performed was a biopsy are 276; the analysis was performed on 2194 patients in whom surgical resection of different grade was accomplished. First author, year of publication, design type, sample size analyzed, and quality and evidence grading data of the studies are presented in Tables 1 and 2.
Table 1.
General characteristics of analyzed publications.
| Study | Year | Design | Limitations | a priori level of evidence | Directness | Imprecision | Confounding variables controlled | Intention to treat principle |
| Ewelt et al[43] | 2011 | Observational data | Serious limitation‡,∗ | Low | uncertain | yes | no | no |
| Oszvald et al[49] | 2012 | Observational data | Serious limitation‡,∗ | Low | uncertain | no | no | no |
| Salvati et al[50] | 2012 | Observational data | Serious limitation‡,∗ | Low | uncertain | yes | no | no |
| Stummer et al[31] | 2012 | Observational data | Serious limitation‡ | Low | uncertain | no | yes | no |
| Shields et al[52] | 2015 | Observational data | Serious limitation‡,∗ | Low | uncertain | yes | no | no |
| Coburger et al[42] | 2015 | Observational data | Serious limitation‡,∗ | Low | uncertain | no | no | no |
| Guden et al[46] | 2016 | Observational data | Serious limitation‡,∗ | Low | uncertain | no | no | no |
| Fukui et al[44] | 2017 | Observational data | Serious limitation‡ | Low | yes | no | yes | no |
| Pessina et al[19] | 2017 | Observational data | Serious limitation‡,∗ | Low | yes | no | no | no |
| Haj et al[47] | 2017 | Observational data | Serious limitation‡,∗ | Low | yes | no | no | no |
| Jiang et al[48] | 2017 | Observational data | Serious limitation‡ | Low | yes | no | yes | no |
| Sharma et al[51] | 2018 | Observational data | Serious limitation‡,∗ | Low | uncertain | yes | no | no |
| Blomstergren et al[41] | 2019 | Observational data | Serious limitation‡ | Low | uncertain | yes | yes | no |
| Gessler et al[45] | 2019 | Observational data | Serious limitation‡ | Low | yes | no | yes | no |
Paper does not discuss reason for not achieving total resection.
No subgroup analysis regarding adjuvant treatment.
Table 2.
Bias characteristics of analyzed publications.
| Bias | ||||||
| Study | Year | Selection and performance | Detection | Attrition | Elements to enhance evidence level | a posteriori Grading of evidence |
| Ewelt et al[43] | 2011 | yes | yes | no | no | very low |
| Oszvald et al[49] | 2012 | yes | yes | no | no | very low |
| Salvati et al[50] | 2012 | yes | yes | no | no | very low |
| Stummer[31] | 2012 | yes | no | no | no | very low |
| Shields et al[52] | 2015 | yes | no | no | no | very low |
| Coburger et al[42] | 2015 | yes | yes | no | no | very low |
| Guden et al[46] | 2016 | yes | yes | no | no | very low |
| Fukui et al[44] | 2017 | yes | yes | yes | no | very low |
| Pessina et al[19] | 2017 | yes | yes | no | no | very low |
| Haj et al[47] | 2017 | yes | yes | no | no | very low |
| Jiang et al[48] | 2017 | yes | no | no | no | very low |
| Sharma et al[51] | 2018 | yes | yes | no | no | very low |
| Blomstergren et al[41] | 2019 | yes | yes | yes | no | very low |
| Gessler et al[45] | 2019 | yes | no | no | no | very low |
Two studies reported on prospective cohorts,[45,49] 2 compared prospective cohorts with historical controls,[31,42] and the rest reported on retrospective cohorts.[19,41,43,44,46–48,50–52]
In this systematic search, we have not found works with quantitative measures of survival in GBM; all the studies included in this work publish their result as Kaplan--Meier survival graphics, the standard way of reporting survival in oncologic studies,[62] making the outcome variable a nominal dichotomic one: alive or dead, for each of the cutting time limits of our research: 12 and 24 months.
The initial level of evidence was low because the included studies were observational investigations in all cases,[63] the quality of evidence in all 14 studies can be degraded to very low, according to GRADE CRITERIA,[53,54] because of a lack of random sequence generation, allocation concealment, and blinding of patients, which leads to a high risk of selection and performance bias[58,59,64]; only 4 studies reported blinding of the evaluators,[31,45,48,52] placing the other 10 at risk of detection bias[65]; 2 articles reported the loss of patients during follow-up,[41,44] putting them at risk of attrition bias[57]; 2 studies comparing retrospective cohorts with historical controls[31,42] increased the risk of selection bias. Two studies included in their statistical analysis propensity-score matched or other matching technique,[45,48] 3 used group-adjustment techniques,[31,41,44] the other 9 did not use any statistical method to amend the bias that confounding variables may induce; none of the studies sustain an intention to treat principle.
Neither one of the papers holds elements to enhance its evidence level.[53,64]
Five studies searched for an association between the amount of resection and overall survival as primary, explicit, and formal objective,[19,44,45,47,48] and have clear outcome measures similar to those of interest in our analysis; the other nine studies had different main objectives, so directness is uncertain.[54]
All studies included in their analysis patients with GBM located in different anatomical areas of dissimilar forms and sizes. None of the studies limit its analysis to tumors in which complete resection is possible and likely as seen in the preoperative evaluation. Adjuvant treatment diverges between papers; 5 studies inform about the Stupp protocol application.[19,31,46,50,52] The surgical protocol has dissimilarity between studies; 10 papers do not specify if neuronavigation was used during the operation;[31,41,43–46,49–52] trans-operatory use of ultrasound is defined in 2 papers,[19,48] use of trans-operatory neurophysiology records in 3,[19,42,48] visualization aid with fluorescence with 5-aminolevulinic acid (5-ALA) in 2,[43,47] and trans-operatory use of MRI are detailed in 2 papers.[42,44]
Six (43%) of the 14 studies included in their analysis patients who did not undergo surgical resection, but a biopsy,[19,43,46,49,50,52] the reason is not explained. In these 6 studies, the percentage of patients that just underwent a biopsy instead of surgical resection goes from 13% to 41%.
All papers report the completion of at least 1 preoperative MRI.
Eight studies included the MGMT methylation status among their variables,[19,31,41,42,45,47,50,51] but 6 did not consider it.
There is a great amount of variation in the maximum level of resection reported: 8 studies state 100% of resection as their foremost goal,[19,31,43–45,47,49,50] one 98%,[48] two 95%,[41,42] one 86%,[51] and 2 studies report gross total resection (GTR) without a numerical description of their definition.[46,52] There are different characterizations in the eight studies that report 100% of tumor resection as their objective, on what they mean like 100% of resection. The definition coincides in 2 of them: (preoperative volume – post-operative volume / preoperative volume) × 100%,[19,51] and in 2 of them: (preoperative tumoral volume – postoperative tumoral volume) / preoperative tumoral volume.[44,48]
The proportion of patients in whom the goal of resection is achieved is also highly variable; it goes from 7% to 86%, with an average of 47%.
Overall survival interval goes from 5 to 33 months; 60% of the studies report mean overall survival between 12 and 18 months. The mean of the 14 studies is 14.3 months (SD = 1.62; CI 95% 13.8–15.6).
4.1. Heterogeneity
DerSimonian and Laird's test for heterogeneity produced I2 value of 29.5409 (P = .01) for 12 months overall survival analysis. The studies with more considerable heterogeneity contribution are from Gessler et al,[45] Pessina et al,[19] and Salvati et al.[50]
In the 24-months overall survival analysis, the DerSimonian and Laird's test for heterogeneity I2 value was 60.09 (P < .01). The studies with more considerable heterogeneity contributions are the ones from Coburger et al,[42] Fukui et al,[44] and Pessina et al.[19]
Figure 2 shows the Galbraith plot of meta-analysis for 12 and 24-months survival.
Figure 2.
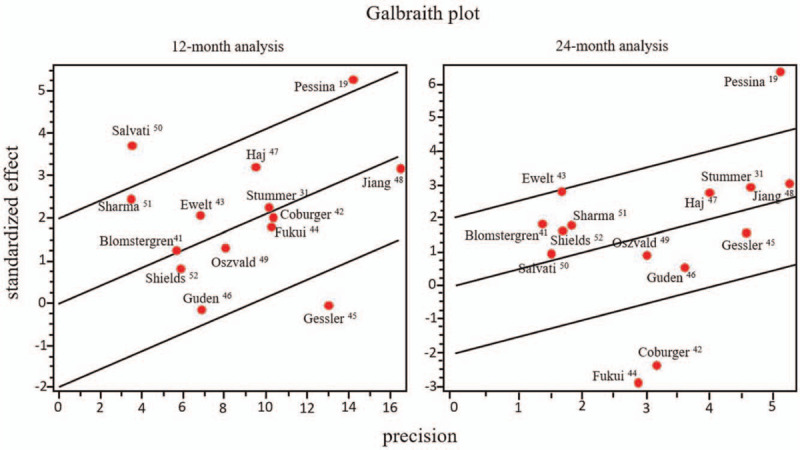
Galbraith plot for meta-analysis for overall survival at 12 and 24 months.
A model of individual and combined results without these studies was also performed.
4.2. Publication bias and sensibility
We did not find statistical evidence of publication bias in the 12-months overall survival analysis (Begg test, Z = 1.0949, P = .27; Egger test, t = 1.2256, P = .24); none of the studies substantially modifies the global result if eliminated from the meta-analysis. Meanwhile, Begg test Z is 0.3285, P = .74 in the 24-months overall survival analysis; the most influential papers in this analysis are the ones by Coburger et al, [42] Ewelt et al,[43] Fukui et al,[44] and Pessina et al.[19]
Funnel plot graphic for 12- and 24-month analyses is shown in Figure 3.
Figure 3.
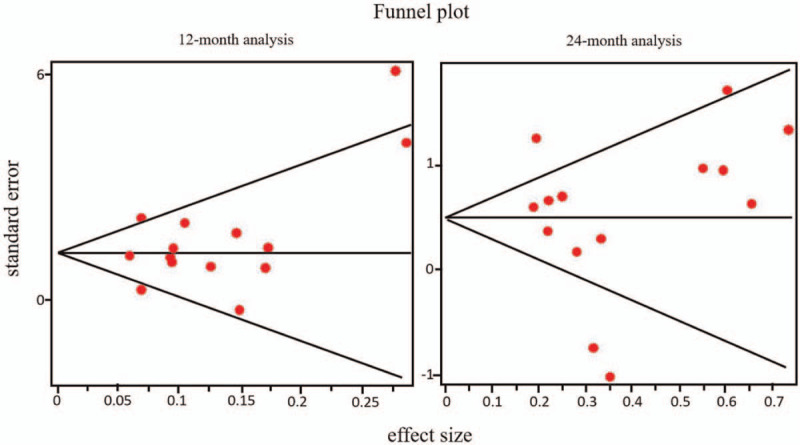
Funnel plot graphic for 12 and 24-month analyses.
4.3. Individual and combined results
4.3.1. Twelve-month analysis
The individual and combined RR results for the 12-months overall survival analysis are summarized in Table 3; 95% CIs and the probability of RR being equal to 1 are included. Those for 24-months overall survival analysis are summarized in Table 4. Forest plots for individual and combined results for 12- and 24-months analyses are shown in Figures 4 and 5, respectively.
Table 3.
Individual and combined results for 12-months analysis.
| Study | Year | n | RR | (95.0% CI) | P | |
| Ewelt et al[43] | 2011 | 60 | 1.34 | 1.01 | 1.78 | .06 |
| Oszvald et al[49] | 2012 | 234 | 1.17 | 0.91 | 1.49 | .24 |
| Salvati et al[50] | 2012 | 80 | 2.80 | 1.63 | 4.82 | .00 |
| Stummer et al[31] | 2012 | 143 | 1.24 | 1.03 | 1.50 | .02 |
| Shields et al[52] | 2015 | 62 | 1.14 | 0.82 | 1.60 | .41 |
| Coburger et al[42] | 2015 | 177 | 1.21 | 1.00 | 1.46 | .07 |
| Guden et al[46] | 2016 | 80 | 0.98 | 0.74 | 1.29 | .87 |
| Fukui et al[44] | 2017 | 168 | 1.19 | 0.99 | 1.44 | .06 |
| Pessina et al[19] | 2017 | 224 | 1.44 | 1.26 | 1.66 | .01 |
| Haj et al[47] | 2017 | 149 | 1.39 | 1.13 | 1.70 | .00 |
| Jiang et al[48] | 2017 | 416 | 1.21 | 1.07 | 1.36 | .00 |
| Sharma et al[51] | 2018 | 156 | 2.01 | 1.15 | 3.53 | .00 |
| Blomstergren et al[41] | 2019 | 70 | 1.25 | 0.89 | 1.76 | .18 |
| Gessler et al[45] | 2019 | 175 | 0.99 | 0.86 | 1.16 | .94 |
| 2194 | ||||||
| Combined (random-effects) | 1.2468 | 1.1404 | 1.3632 | .00 | ||
P: probability of RR being equal to 1. CI = confidence interval; RR = relative risk.
If the publications by Pessina, Salvati, and Gessler[19,45,50] are withdrawn from the analysis because of their heterogeneity contribution, effect size for fixed-effects model diminishes to 1.23 but remains with statistical significance (95% CI 1.147--1.305, P = .00).
Table 4.
Individual and combined results for 24-months analysis.
| Study | Year | n | RR | (95.0% CI) | P | |
| Ewelt et al[43] | 2011 | 60 | 5.36 | 1.65 | 17.46 | .00 |
| Oszvald et al[49] | 2012 | 234 | 1.33 | 0.69 | 2.55 | .40 |
| Salvati et al[50] | 2012 | 80 | 1.81 | 0.51 | 6.52 | .50 |
| Stummer et al[31] | 2012 | 143 | 1.86 | 1.22 | 2.83 | .00 |
| Shields et al[52] | 2015 | 62 | 2.53 | 0.79 | 8.04 | .09 |
| Coburger et al[42] | 2015 | 177 | 0.46 | 0.25 | 0.86 | .01 |
| Guden et al[46] | 2016 | 80 | 1.16 | 0.67 | 2.00 | .59 |
| Fukui et al[44] | 2017 | 168 | 0.35 | 0.18 | 0.69 | .00 |
| Pessina et al[19] | 2017 | 224 | 3.45 | 2.36 | 5.06 | .00 |
| Haj et al[47] | 2017 | 149 | 1.97 | 1.21 | 3.21 | .01 |
| Jiang et al[48] | 2017 | 416 | 1.76 | 1.22 | 2.54 | .00 |
| Sharma et al[51] | 2018 | 156 | 2.57 | 0.88 | 7.55 | .05 |
| Blomstergren et al[41] | 2019 | 70 | 3.75 | 0.89 | 15.87 | .06 |
| Gessler et al[45] | 2019 | 175 | 1.40 | 0.91 | 2.14 | .15 |
| 2194 | ||||||
| Combined (random-effects) | 1.59 | 1.11 | 2.26 | .00 | ||
P: probability of RR being equal to 1.
If the publications by Coburger, Fukui, and Pessina[19,42,44] are withdrawn from the analysis because of their heterogeneity contribution, effect size for fixed-effects model yields 1.68, but remains with statistical significance (95% CI 1.14--1.99, P = .00).
CI = confidence interval; RR = relative risk.
Figure 4.
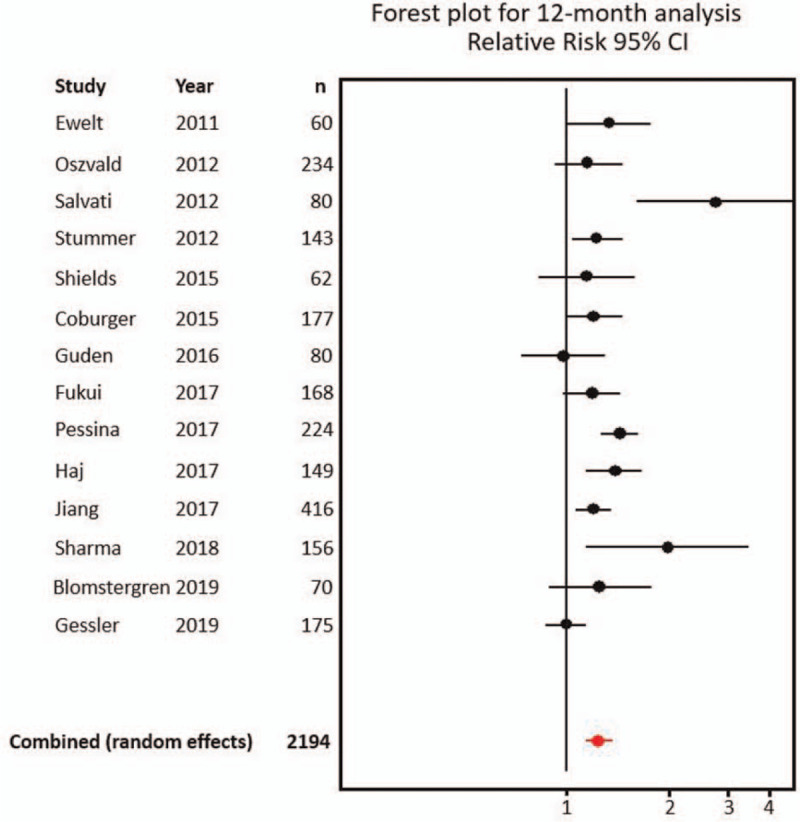
Forest plot for individual and combined results at 12 months. CI = confidence interval.
Figure 5.
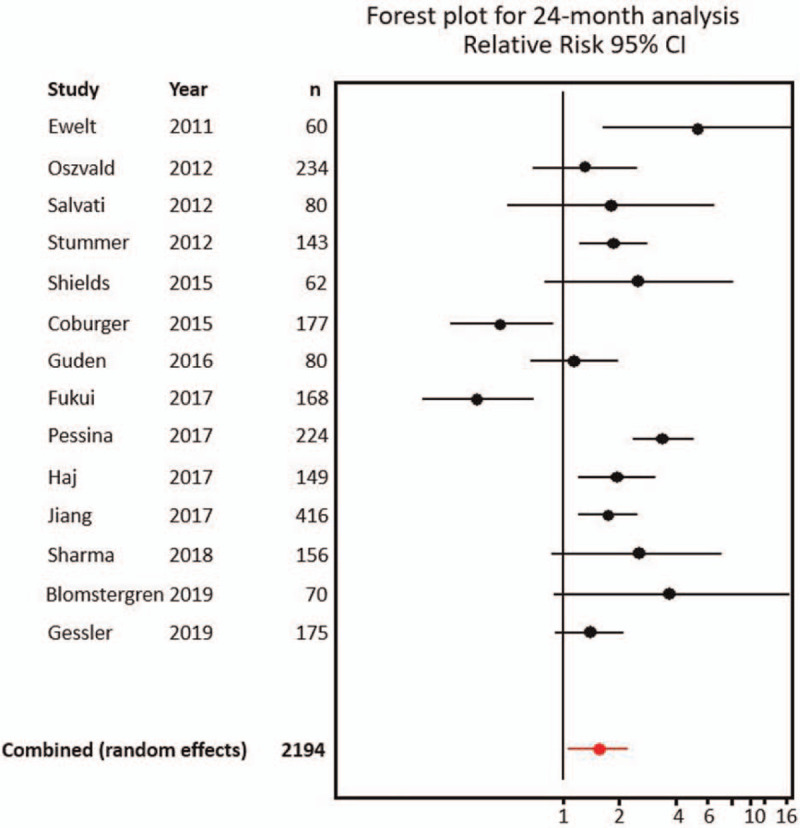
Forest plot for individual and combined results at 24 months. CI = confidence interval.
The largest analyzed sample studies are the ones from Jiang et al (416 patients analyzed),[48] Oszvald et al (234 patients analyzed),[49] and Pessina et al (224 patients analyzed).[19] The ones with the smallest analyzed samples are Ewelt et al (60 patients analyzed),[43] Shield et al (62 patients analyzed),[52] and Blomstergren et al (70 patients analyzed).[41] Pearson correlation coefficient for sample size and OR for 12-months analysis is -0.185, P = .53; for 24-months analysis is -0.316, P = .27.
For the 12-months analysis, we found a lack of consistency in estimates of effects across studies: RR goes from 0.98 (95% CI 0.74--1.29) in study by Guden et al[46] to 2.80 (95% CI 1.63--4.82) in study by Salvati et al.[50] Six of 14 studies (43%) show a statistically significant association between more extensive resection and better overall survival[19,31,47,48,50,51]; 6 show a weak association without statistical significance,[41–44,49,52] and 2 show a weak association between subtotal resection and better survival, without statistical significance.[45,46]
Effect size for random-effects model for the association between total resection and overall survival for 12-months analysis is 1.25 (95% CI 1.14–1.36, P < .01), ARR of 15.7% (95% CI 11.9–19.4), RRR of 0.25 (95% CI 0.18–0.31), NNT 6.
If the publications by Gessler et al, [45] Pessina et al, [1] and Salvati et al[50] are withdrawn from the analysis because of their heterogeneity contribution, the effect size for the fixed-effects model diminishes to 1.22 but remains with statistical significance (95% CI 1.147–1.305, P < .01).
If the analysis is reduced to 5 papers with explicit directness regarding the search for an association between the amount of resection and overall survival,[19,44,45,47,48] the effect size for the random-effects model remains 1.23 with statistical significance (95% CI 1.079–1.405, P < .01).
4.3.2. Twenty-four-month analysis
For the 24-months analysis, we found a lack of consistency in estimates of effects across studies: RR goes from 0.35 (95% CI 0.18–0.69) in study by Fukui et al,[44] to 5.36 (95% CI 1.65–17.46) in study by Ewelt et al.[43] Five of 14 studies show a statistically significant association between more extensive resection and better overall survival[19,31,43,47,48]; 7 show a weak association without statistical significance,[41,45,46,49–52] and 2 show a strong association between subtotal resection and better survival, with statistical significance.[42,44]
The effect size for the random-effects model for the association between total resection and overall survival for 24-months analysis is 1.59 (95% CI 1.11–2.26, P < .01), ARR is 11.5%, RRR of 0.53, and NNT 9.
If the papers that contribute the most to heterogeneity---the ones by Coburger et al,[42] Fukui et al,[44] and Pessina et al[19]---are subtracted from the analysis, fixed-effects model yields combined RR for the association between total resection and overall survival for 24 months of 1.716 (CI 95% 1.44–2.05, P < .01).
If the analysis is reduced to 5 papers with explicit directness regarding the search for an association between the amount of resection and overall survival,[19,44,45,47,48] the effect size for the random-effects model diminishes to 1.49 and loses its statistical significance (95% CI 0.824–2.685, P = .15).
4.3.3. Cohens d effect size for 12- and 24-month analyses
As Chen et al[66] have proposed, the size of RR can be transformed to a Cohen d equivalence by relating it to differences in a normal standard deviate. As such, our findings of RR 1.25 at 12 and 1.59 at 24 months are equivalent to a low (Cohen d = 0.2) Cohen d effect.
According to GRADE criteria, all 14 studies from which this study obtained the analyzed data sustain a very low level of evidence.
5. Discussion
Given the ominous prognosis in patients with GBM, factors that may extend survival are being extensively searched for. Several factors have been proposed as predictive of overall survival prognosis; of these, only the extent of surgical resection, residual volume, and adjuvant oncological treatment are direct modifiable factors.[9,38,41,67–69]
To date, there is no consensus on how much tumor should be resected to provide a better prognosis,[2,15,19,24,26] current treatment guidelines recommend maximum safe resection,[8] leaving the interpretation of the recommendation to the team treating the patient.
The best possible conclusion that statistical methods can obtain comes from meta-analyses of controlled clinical trials in which the main outcome is a quantitative one, derived from data with normal distribution, which provides the greater possible power,[70–72] but no randomized controlled clinical trial has attempted to answer the question, and an appropriate design is not feasible,[31] because of this, current attempts to correctly answer the question are based on scanty studies of limited designs, with nominal outcome variables, that produce a low or very low level of evidence.[53,54]
In the search for the appropriate answer to this issue, we have found in a meta-analysis of 14 heterogeneous studies with a very low level of evidence according to GRADE Criteria[53,54,64,73] that when diagnosed with a GBM, patients have 15.7% more risk to die at 12 months if a subtotal surgical resection of the tumor has been performed, and 11.5% more risk to die at 24 months in the same situation; RR to be alive at 12 months with maximal resection is 1.25 (95% CI 1.14–1.36, P < .01), at 24 months it is 1.58 (95% CI 1.11–2.26, P < .01). Our findings of RR 1.25 at 12 and 1.59 at 24 months are equivalent to a low Cohen d effect of 0.2.[66]
It is noteworthy the lack of consistency we found: in the 12-month analysis, 2 studies show a nonstatistically significant association between subtotal resection and better prognosis[45,46]; in the 24-month analysis, this finding reaches statistically significant association in the studies by Coburger et al[42] and Fukui et al.[44]
When the analysis is limited to 5 studies with explicit directness regarding our main research question,[19,44,45,47,48] the effect size for the random-effects model diminishes to 1.49 and loses its statistical significance (95% CI 0.824–2.685, P = .15) in the 24-month analysis.
The results we found should be cautiously considered because several factors impede reaching a simple conclusion.
One of the main issues is that to this date, no randomized controlled clinical trial has attempted to answer the question, and an appropriate design is not feasible:[31] to randomize patients with similar characteristics and assign them blindly to different predetermined extents of resection. Because of this, the attempt to properly answer the question needs to be based on scanty studies that produce a very low level of evidence.[53,54]
A Medline search for meta-analysis regarding 12 and 24 months overall survival related to the extent of resection in GBM patients produces 2 direct[7,74] and 1 indirect results.[75] The formers analyze the association between the extent of resection and survival, as we did. The latter one examines the use of intraoperative MRI in aid of complete surgical resection and its impact on survival.
Results for study by Brown et al[7] (transformed for matching our research question) are: number of studies analyzed = 37, total number of patients = 41117, pooled RR for 12 months = 1.61 (95% CI 1.44–1.78, P < .01); RR for 24 months = 1.19 (95% CI 1.12–1.26, P < .01).
Results from study by Li et al[74] are number of studies analyzed = 6, total number of patients = 1618, pooled RR for 12 months = RR, 1.89; 95% CI 1.35–2.64, P < .01. No results for 24 months.
Even when the number of studies and patients analyzed differ between them and also from ours, pooled RR results are similar to the ones obtained in our study.
In the case of study by Li et al,[75] noteworthily, they found that the use of intraoperative MRI was associated with a greater rate of GTR compared with conventional neuronavigation procedures (OR 3.16, 95% CI 2.07–4.83, P < .01); nonetheless, they found that the overall survival at 12 months between groups has no statistically significant difference (P = .80).
The percentage of resection associated with better survival time when it occurs is highly variable, ranging from 75% to 100% in the literature and from 86% to 100% in this study. The studies that conclude an association between 100% resection and better overall survival, if true, would arguably invalidate the results of any other research that has found an association between the lower percentage of resection and longer survival time.
The predictive resection cut-off value consistently associated with longer survival time cannot be defined at this time.
This concept opens up an area of research opportunity on factors that could act as confounding variables, such as the possibility that tumors suited to complete resection are associated with a better prognosis than are highly infiltrative tumors in eloquent brain areas, the relationship of tumors with neurogenic areas,[76] tumor shape,[32] or improved functional status from surgical decompression and its association with better overall survival.[48,77]
Given that there is a current tendency to consider that the methylation state of the MGMT promoter is the strongest predictor of outcomes in GBM treatment,[46,78] we suggest that future studies that analyze the association between the extent of resection and overall survival in GBM should include that analysis for all cases.
5.1. Limitations
The main limitation of this study is that the analysis is based on descriptive studies, primarily retrospective, nonrandomized, and unblinded cohorts, in which management protocols are highly variable, which produce very low level of evidence, in consequence, any estimate of effect is very uncertain.
Another limitation is the lack of statistical methods aimed at reducing the impact of confounding variables on the results. Even when the statical procedures were adequately performed in order to detect reporting bias and no evidence of it was found, we are aware of the possibility of incomplete retrieval of identified research.
6. Conclusion
The relative risk combined result for the association between total resection and overall survival for 12-months analysis we have found in this meta-analysis is 1.25 (95% CI 1.14--1.36, P < .01), which augments to 1.58 (95% CI 1.111--2.262, P < .01) and remains statistically significant for the 24-months analysis. In both cases, the results support a more extensive over subtotal resection in association with overall survival for GBM.
The results imply a weak correlation obtained from studies with a very low level of evidence, according to GRADE Criteria; in consequence, any estimate of effect is very uncertain,[53,54] and do not necessarily conclude that there is a cause-and-effect relationship.
Despite the difficulty of designing a study aimed at adequately answering the question of whether complete resection of GBM leads to a consistent increase in overall survival, future research will likely have a significant impact on the estimation of the effect, which is currently uncertain.[53]
The statistical analysis has been performed by Francisco Revilla-Pacheco, PhD, Postgraduate Professor of Biostatistics, Universidad Anahuac College of Medicine.
Author contributions
Conceptualization: Francisco Revilla-Pacheco.
Investigation: Pamela Rodríguez-Salgado, Mónica Barrera-Ramírez, Maria Paula Morales-Ruiz, Tenoch Herrada-Pineda.
Methodology: Francisco Revilla-Pacheco.
Validation: Francisco Revilla-Pacheco, Johnatan Rubalcava-Ortega, Tenoch Herrada-Pineda.
Visualization: Francisco Revilla-Pacheco, Mauro Loyo-Varela.
Writing – original draft: Pamela Rodríguez-Salgado.
Writing – review & editing: Pamela Rodríguez-Salgado.
Footnotes
Abbreviations: ARR = absolute risk reduction, CENTRAL = Cochrane Central Register of Controlled Trials, CI = confidence interval, ESR = extent of surgical resection, GBM = glioblastoma multiforme, GRADE = Grading of Recommendations Assessment, Development, and Evaluation, LILACS = Latin American and Caribbean Health Sciences Literature, MGMT = O6-methylguanine-methyltransferase, MRI = magnetic resonance imaging, NNT = number needed to treat, OR = odds ratio, OS = overall survival, PRISMA-P = Preferred reporting items for systematic review and meta-analysis protocols, RR = relative risk, RRR = relative risk reduction, SD = standard deviation.
How to cite this article: Revilla-Pacheco F, Rodríguez-Salgado P, Barrera-Ramírez M, Morales-Ruiz MP, Loyo-Varela M, Rubalcava-Ortega J, Herrada-Pineda T. Extent of resection and survival in patients with glioblastoma multiforme: systematic review and meta-analysis. Medicine. 2021;100:25(e26432).
No funding was received for this research.
The protocol was presented to the Anahuac University Research and Ethics Committee; it was accepted for its development on November 2019, with acceptance number 1371919.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with human participants performed by any of the authors.
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Ostrom QT, Gittleman H, Xu J, et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol 2016;18: suppl_5: v1–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- [3].Sanai N, Polley MY, McDermott MW, et al. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 2011;115:03–8. [DOI] [PubMed] [Google Scholar]
- [4].Oppenlander ME, Wolf AB, Snyder LA, et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg 2014;120:846–53. [DOI] [PubMed] [Google Scholar]
- [5].Chambless LB, Kistka HM, Parker SL, et al. The relative value of postoperative versus preoperative Karnofsky Performance Scale scores as a predictor of survival after surgical resection of glioblastoma multiforme. J Neuro-oncol 2015;121:359–64. [DOI] [PubMed] [Google Scholar]
- [6].Jiang S, Hill K, Patel D, et al. Direct medical costs of treatment in newly-diagnosed high-grade glioma among commercially insured US patients. J Med Econ 2017;20:1237–43. [DOI] [PubMed] [Google Scholar]
- [7].Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol 2016;2:1460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nabors LB, Portnow J, Ammirati M, et al. Central nervous system cancers, version 2.2014: featured updates to the NCCN Guidelines. J Natl Comp Cancer Netw 2014;12:1517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Smets T, Lawson TM, Grandin C, et al. Immediate postoperative MRI suggestive of the site and timing of glioblastoma recurrence after gross total resection: a retrospective longitudinal preliminary study. Eur Radiol 2013;23:1467–77. [DOI] [PubMed] [Google Scholar]
- [10].Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 2001;95:190–8. [DOI] [PubMed] [Google Scholar]
- [11].Kuhnt D, Becker A, Ganslandt O, et al. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro Oncol 2011;13:1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ushio Y, Kochi M, Hamada J, et al. Effect of surgical removal on survival and quality of life in patients with supratentorial glioblastoma. Neurol Med Chir 2005;45:454–60. discussion 460-461. [DOI] [PubMed] [Google Scholar]
- [13].Vidiri A, Carapella CM, Pace A, et al. Early postoperative MRI: correlation with progression-free survival and overall survival time in malignant gliomas. J Exp Clin Cancer Res 2006;25:177–82. [PubMed] [Google Scholar]
- [14].Willems PW, Taphoorn MJ, Burger H, et al. Effectiveness of neuronavigation in resecting solitary intracerebral contrast-enhancing tumors: a randomized controlled trial. J Neurosurg 2006;104:360–8. [DOI] [PubMed] [Google Scholar]
- [15].Stummer W, Pichlmeier U, Meinel T, et al. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006;7:392–401. [DOI] [PubMed] [Google Scholar]
- [16].Swanson KR, Rostomily RC, Alvord EC, Jr. A mathematical modelling tool for predicting survival of individual patients following resection of glioblastoma: a proof of principle. Br J Cancer 2008;98:113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Swanson KR, Bridge C, Murray JD, et al. Virtual and real brain tumors: using mathematical modeling to quantify glioma growth and invasion. J Neurol Sci 2003;216:01–10. [DOI] [PubMed] [Google Scholar]
- [18].Li YM, Suki D, Hess K, et al. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg 2016;124:977–88. [DOI] [PubMed] [Google Scholar]
- [19].Pessina F, Navarria P, Cozzi L, et al. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: is it useful and safe? A single institution retrospective experience. J Neuro Oncol 2017;135:129–39. [DOI] [PubMed] [Google Scholar]
- [20].McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J Neurosurg 2009;110:156–62. [DOI] [PubMed] [Google Scholar]
- [21].Rahman M, Abbatematteo J, De Leo EK, et al. The effects of new or worsened postoperative neurological deficits on survival of patients with glioblastoma. J Neurosurg 2017;127:123–31. [DOI] [PubMed] [Google Scholar]
- [22].Li Y, Rey-Dios R, Roberts DW, et al. Intraoperative fluorescence-guided resection of high-grade gliomas: a comparison of the present techniques and evolution of future strategies. World Neurosurg 2014;82:175–85. [DOI] [PubMed] [Google Scholar]
- [23].Maugeri R, Villa A, Pino M, et al. With a little help from my friends: the role of intraoperative fluorescent dyes in the surgical management of high-grade gliomas. Brain Sci 2018;8:02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shinoda J, Yano H, Yoshimura S, et al. Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium. Technical note. J Neurosurg 2003;99:597–603. [DOI] [PubMed] [Google Scholar]
- [25].Koc K, Anik I, Cabuk B, et al. Fluorescein sodium-guided surgery in glioblastoma multiforme: a prospective evaluation. Br J Neurosurg 2008;22:99–103. [DOI] [PubMed] [Google Scholar]
- [26].Pirotte BJ, Levivier M, Goldman S, et al. Positron emission tomography-guided volumetric resection of supratentorial high-grade gliomas: a survival analysis in 66 consecutive patients. Neurosurgery 2009;64:471–81. discussion 481. [DOI] [PubMed] [Google Scholar]
- [27].Poon MT, Sudlow CL, Figueroa JD, et al. Longer-term (≥ 2 years) survival in patients with glioblastoma in population-based studies pre-and post-2005: a systematic review and meta-analysis. Scie Rep 2020;10:01–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Muller DMJ, Robe P, Eijgelaar RS, et al. Comparing glioblastoma surgery decisions between teams using brain maps of tumor locations, biopsies, and resections. JCO Clin Cancer Inform 2019;3:01–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Suchorska B, Weller M, Tabatabai G, et al. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro Oncol 2016;18:549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Albert FK, Forsting M, Sartor K, et al. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 1994;34:45–61. [DOI] [PubMed] [Google Scholar]
- [31].Stummer W, Meinel T, Ewelt C, et al. Prospective cohort study of radiotherapy with concomitant and adjuvant temozolomide chemotherapy for glioblastoma patients with no or minimal residual enhancing tumor load after surgery. J Neuro Oncol 2012;108:89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sanghani P, Ti AB, Kam King NK, et al. Evaluation of tumor shape features for overall survival prognosis in glioblastoma multiforme patients. Surg Oncol 2019;29:178–83. [DOI] [PubMed] [Google Scholar]
- [33].Senft C, Bink A, Franz K, et al. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 2011;12:997–1003. [DOI] [PubMed] [Google Scholar]
- [34].Pichlmeier U, Bink A, Schackert G, et al. Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol 2008;10:1025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Vuorinen V, Hinkka S, Farkkila M, et al. Debulking or biopsy of malignant glioma in elderly people: a randomised study. Acta Neurochir 2003;145:05–10. [DOI] [PubMed] [Google Scholar]
- [37].Xing Y, Wang X. Which parameter is more important for the prognosis of new-onset adult glioblastoma: residual tumor volume or extent of resection? World Neurosurg 2018;116:e444–51. [DOI] [PubMed] [Google Scholar]
- [38].Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol 2014;16:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Familiari P, Frati A, Pesce A, et al. Real impact of intraoperative magnetic resonance imaging in newly diagnosed glioblastoma multiforme resection: an observational analytic cohort study from a single surgeon experience. World Neurosurg 2018;116:e9–17. [DOI] [PubMed] [Google Scholar]
- [40].Pessina F, Navarria P, Cozzi L, et al. Is surgical resection useful in elderly newly diagnosed glioblastoma patients? Outcome evaluation and prognostic factors assessment. Acta Neurochir 2018;160:1779–87. [DOI] [PubMed] [Google Scholar]
- [41].Blomstergren A, Rydelius A, Abul-Kasim K, et al. Evaluation of reproducibility in MRI quantitative volumetric assessment and its role in the prediction of overall survival and progression-free survival in glioblastoma. Acta Radiol (Stockholm, Sweden: 1987) 2019;60:516–25. [DOI] [PubMed] [Google Scholar]
- [42].Coburger J, Hagel V, Wirtz CR, et al. Surgery for glioblastoma: impact of the combined use of 5-aminolevulinic acid and intraoperative MRI on extent of resection and survival. PLoS One 2015;10:e0131872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ewelt C, Goeppert M, Rapp M, et al. Glioblastoma multiforme of the elderly: the prognostic effect of resection on survival. J Neuro Oncol 2011;103:611–8. [DOI] [PubMed] [Google Scholar]
- [44].Fukui A, Muragaki Y, Saito T, et al. Volumetric analysis using low-field intraoperative magnetic resonance imaging for 168 newly diagnosed supratentorial glioblastomas: effects of extent of resection and residual tumor volume on survival and recurrence. World Neurosurg 2017;98:73–80. [DOI] [PubMed] [Google Scholar]
- [45].Gessler F, Bernstock JD, Braczynski A, et al. Surgery for glioblastoma in light of molecular markers: impact of resection and MGMT promoter methylation in newly diagnosed IDH-1 wild-type glioblastomas. Neurosurgery 2019;84:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Guden M, Ayata HB, Ceylan C, et al. Prognostic factors effective on survival of patients with glioblastoma: Anadolu Medical Center experience. Indian J Cancer 2016;53:382–6. [DOI] [PubMed] [Google Scholar]
- [47].Haj A, Doenitz C, Schebesch KM, et al. Extent of resection in newly diagnosed glioblastoma: impact of a specialized neuro-oncology care center. Brain Sci 2017;8:01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jiang H, Cui Y, Liu X, et al. Patient-specific resection strategy of glioblastoma multiforme: choice based on a preoperative scoring scale. Ann Surg Oncol 2017;24:2006–14. [DOI] [PubMed] [Google Scholar]
- [49].Oszvald A, Guresir E, Setzer M, et al. Glioblastoma therapy in the elderly and the importance of the extent of resection regardless of age. J Neurosurg 2012;116:357–64. [DOI] [PubMed] [Google Scholar]
- [50].Salvati M, Pichierri A, Piccirilli M, et al. Extent of tumor removal and molecular markers in cerebral glioblastoma: a combined prognostic factors study in a surgical series of 105 patients. J Neurosurg 2012;117:204–11. [DOI] [PubMed] [Google Scholar]
- [51].Sharma M, Bellamkonda S, Mohapatra S, et al. Correlation between the residual tumor volume, extent of tumor resection, and O(6)-methylguanine DNA methyltransferase status in patients with glioblastoma. World Neurosurg 2018;116:e147–61. [DOI] [PubMed] [Google Scholar]
- [52].Shields LB, Shelton BJ, Shearer AJ, et al. Dexamethasone administration during definitive radiation and temozolomide renders a poor prognosis in a retrospective analysis of newly diagnosed glioblastoma patients. Radiat Oncol (London, England) 2015;10:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Brozek J, Akl E, Jaeschke R, et al. GRADE Working Group: Grading quality of evidence and strength of recommendations in clinical practice guidelines: Part 2 of 3. The GRADE approach to grading quality of evidence about diagnostic tests and strategies. Allergy 2009;64:1109–16. [DOI] [PubMed] [Google Scholar]
- [55].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2011. [Google Scholar]
- [57].Nunan D, Aronson J, Bankhead C. Catalogue of bias: attrition bias. BMJ Evid Based Med 2018;23:21–2. [DOI] [PubMed] [Google Scholar]
- [58].Nunan D, Heneghan C, Spencer EA. Catalogue of bias: allocation bias. BMJ Evid Based Med 2018;23:20–1. [DOI] [PubMed] [Google Scholar]
- [59].Higgins JP. Cochrane handbook for systematic reviews of interventions. Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration. www.cochrane-handbook.org, 2011. [Google Scholar]
- [60].Kontopantelis E, Springate DA, Reeves D. A re-analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta-analyses. PLoS One 2013;8:e69930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Cheung MW, Ho RC, Lim Y, et al. Conducting a meta-analysis: basics and good practices. Int J Rheum Dis 2012;15:129–35. [DOI] [PubMed] [Google Scholar]
- [62].Goel MK, Khanna P, Kishore J. Understanding survival analysis: Kaplan-Meier estimate. Int J Ayurveda Res 2010;1:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Atkins D, Eccles M, Flottorp S, et al. Systems for grading the quality of evidence and the strength of recommendations I: critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv Res 2004;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gross RA, Johnston KC. Levels of evidence: taking neurology to the next level. Neurology 2009;72:08–10. [DOI] [PubMed] [Google Scholar]
- [65].Mahtani K, Spencer EA, Brassey J, et al. Catalogue of bias: observer bias. BMJ Evid Based Med 2018;23:23–4. [DOI] [PubMed] [Google Scholar]
- [66].Chen H, Cohen P, Chen S. How big is a big odds ratio? Interpreting the magnitudes of odds ratios in epidemiological studies. Commun Stat Simul Comput 2010;39:860–4. [Google Scholar]
- [67].Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol 2003;30: 6 Suppl 19: 10–4. [DOI] [PubMed] [Google Scholar]
- [68].Weller M, Tabatabai G, Kastner B, et al. MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR Trial. Clin Cancer Res 2015;21:2057–64. [DOI] [PubMed] [Google Scholar]
- [69].Grabowski MM, Recinos PF, Nowacki AS, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg 2014;121:1115–23. [DOI] [PubMed] [Google Scholar]
- [70].Rutka JT. Classes of evidence in neurosurgery. J Neurosurg 2016;126:1747–8. [DOI] [PubMed] [Google Scholar]
- [71].Sprague S, Pozdniakova P, Kaempffer E, et al. Principles and practice of clinical research course for surgeons: an evaluation of knowledge transfer and perceptions. Can J Surg 2012;55:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].2011;Bhandari M, Joensson A. Clinical research for surgeons Thieme. [Google Scholar]
- [73].Group GW. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Li XZ, Li YB, Cao Y, et al. Prognostic implications of resection extent for patients with glioblastoma multiforme: a meta-analysis. J Neurosurg Sci 2017;61:631–9. [DOI] [PubMed] [Google Scholar]
- [75].Li P, Qian R, Niu C, et al. Impact of intraoperative MRI-guided resection on resection and survival in patient with gliomas: a meta-analysis. Curr Med Res Opinion 2017;33:621–30. [DOI] [PubMed] [Google Scholar]
- [76].Mistry AM, Dewan MC, White-Dzuro GA, et al. Decreased survival in glioblastomas is specific to contact with the ventricular-subventricular zone, not subgranular zone or corpus callosum. J Neuro Oncol 2017;132:341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol 2008;9:29–38. [DOI] [PubMed] [Google Scholar]
- [78].Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–66. [DOI] [PubMed] [Google Scholar]


