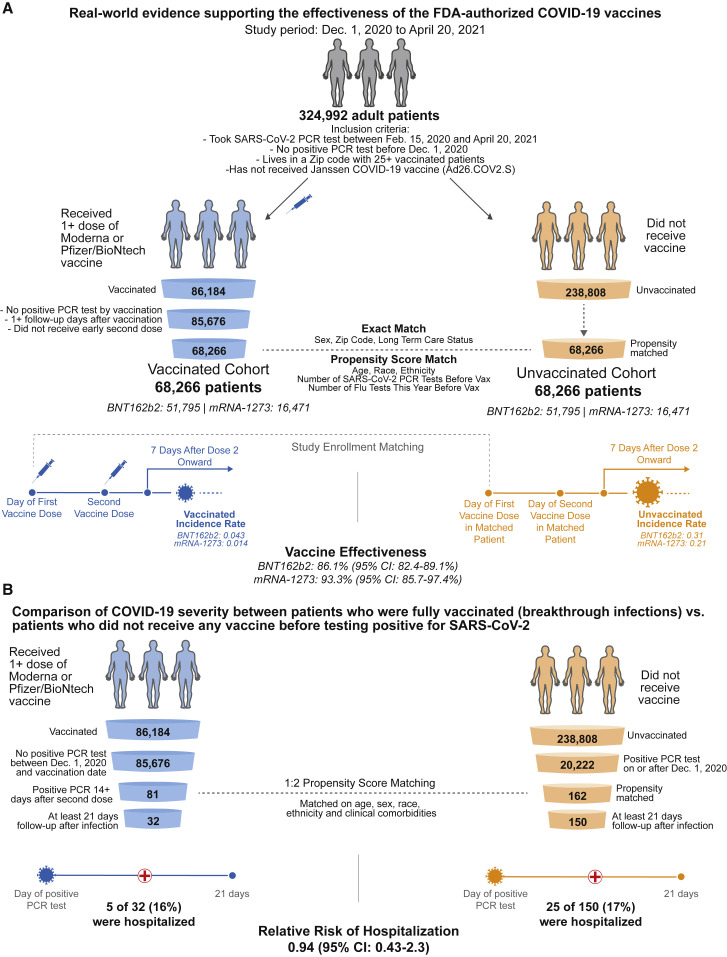
Figure 1.
Schematic illustrating the algorithms for participant selection and outcome assessment
(A) Design of the study to compare SARS-CoV-2 infection rates in individuals receiving BNT162b2 or mRNA-1273 vaccination compared with 1-to-1 propensity-matched unvaccinated individuals (nBNT162b2 = 51,795 per group; nmRNA-1273 = 16,471 per group). For each group, incidence rates were calculated to assess the effectiveness of vaccination in preventing a positive SARS-CoV-2 PCR test at least 7 days after the second vaccine dose. Several other time windows were also evaluated for vaccine effectiveness.
(B) Design of the study to compare COVID-19 disease severity in individuals who were fully vaccinated at least 14 days prior to diagnosis with COVID-19 (n = 81) and had at least 21 days of follow-up after diagnosis (n = 32) versus 1-to-2 propensity-matched unvaccinated individuals (n = 162) with at least 21 days of follow-up (n = 150). Hospitalization and ICU admission were assessed within 21 days of PCR diagnosis, and mortality was assessed within 28 days of PCR diagnosis.