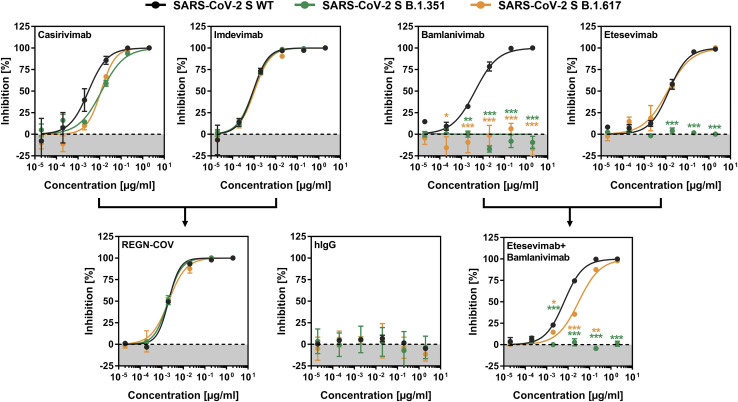
Figure 4.
The S protein of SARS-CoV-2 variant B.1.617 is resistant to neutralization by bamlanivimab
S protein-bearing particles were incubated with different concentrations of monoclonal antibodies for 30 min at 37°C before the mixtures were inoculated onto Vero cells. Transduction efficiency was quantified by measuring virus-encoded luciferase activity in cell lysates 16–18 h after transduction. For normalization, SARS-CoV-2 S protein-driven entry in the absence of monoclonal antibody was set as 0% inhibition. Presented are the average (mean) data from three biological replicates (each performed with technical quadruplicates). Error bars indicate the SEM. Statistical significance of differences between the WT and the variant S proteins was analyzed by two-way ANOVA with Dunnett’s posttest (p > 0.05, ns [not significant, not indicated in the graphs]; ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001). See also Figure S2.