Abstract
Located medially within the temporal lobes, the amygdala is a formation of heterogenous nuclei that has emerged as a target for investigations into the neural bases of both primitive and complex behaviors. Although modern neuroscience has eschewed the practice of assigning broad functions to distinct brain regions, the amygdala has classically been associated with regulating negative emotional processes (such as fear or aggression), primarily through research performed in rodent models. Contemporary studies, particularly those in non-human primate models, have provided evidence for a role of the amygdala in other aspects of cognition such as valuation of stimuli or shaping social behaviors. Consequently, many modern perspectives now also emphasize the amygdala’s role in processing positive affect and social behaviors. Importantly, several recent experiments have examined the intersection of two seemingly autonomous domains; how both valence/value and social stimuli are simultaneously represented in the amygdala. Results from these studies suggest that there is an overlap between valence/value processing and the processing of social behaviors at the level of single neurons. These findings have prompted researchers investigating the neurophysiological mechanisms underlying social interactions to question what contributions reward-related processes in the amygdala make in shaping social behaviors. In this review, we will examine evidence, primarily from primate neurophysiology, suggesting that value-related processes in the amygdala interact with the processing of social stimuli, and explore holistic hypotheses about how these amygdalar interactions might be instantiated.
1. Introduction
The amygdala is a collection of nuclei, conserved across species, that has been implicated in a diverse assortment of behaviors. A large body of literature has established a fundamental role for the amygdala in regulating defensive behaviors such as fear and vigilance [1–3]. Modern techniques in rodent models have enabled the precise dissection of specific amygdala circuits which underlie the acquisition, expression, and extinction of conditioned fear behaviors [4,5].
Beyond representing aversive stimuli or negatively-valenced emotions, the amygdala has been implicated in evaluating rewarding outcomes as well [6–10]. Neurons in both the rodent and primate amygdala encode stimuli associated positive or negative outcomes [9,11–22]. Considering this, it is unsurprising that lesions of the amygdala interfere with the ability of non-human primates to update stimulus-reward associations after devaluation [23] and modulate reward-value coding in other brain areas [24,25], supporting the theory that the amygdala works in conjunction with prefrontal cortical areas during stimulus-reward learning [13,25,26].
In addition to processing both positive and negatively associated stimuli, the amygdala is also well-established in mediating social perception and social decision-making [27–31]. Selective calcification lesions of the amygdala in human patients impairs recognition of emotion in facial expressions [32] and reduces eye contact during social interactions [33]. Neurons that encode the perception of faces [34,35], facial identity and expression [36], eye contact [37,38], auditory social cues [39], and social decisions [28] have been identified in the primate amygdala. This evidence supports the view that the amygdala processes social stimuli and functions together with a network of other structures, such as prefrontal cortex, to coordinate social behaviors [30,31,40].
To varying degrees, each of the above behavioral categories are constructs imposed by experimental designs. For example, few stimuli capture attention more effectively than stimuli that signal danger, which has resulted in ‘fear conditioning’ paradigms to become a staple experimental model of amygdala function, yet the definition of fear itself in relation to the amygdala is still under examination [41,42]. Likewise, many recent experiments have centered around the exploration of explicit value representations in the brain, although alterative interpretations of these neural signals may better explain amygdala functions more holistically [43–46]. Furthermore, some experiments have analyzed social stimuli such as the indirect or direct gaze of a conspecific within the framework of positively or negatively valenced signals [47], while others have argued that these behavioral signals are highly context-dependent [48]. Alternatively it could be argued that these seemingly distinct functions are just incarnations of a broader role for the amygdala in maintaining vigilance and allocating attention to stimuli of importance [49]. There is also debate if social perception and cognition are instantiated in a discrete neural system [50], or has layers of social specificity while simultaneously sharing other processes with non-social aspects of cognition [51]. These contrasting views of the amygdala (and more broadly, the brain) are exacerbated by practical limitations; each individual study is restricted in the range of stimuli, the behavioral states evoked by task designs, or observable behaviors that a study can explore, and consequently there are sparse data that span across these experimentally imposed domains. Recently, however, several studies have examined a parallel question to those posed above: do single neurons in the amygdala have specific functional specializations, or do they exhibit selectivity to multiple dimensions? Evidence increasingly supports that, indeed, neurons in the amygdala do exhibit complex multidimensional responses, a perspective which has been recently explored in depth by Gothard (2020). The purpose of the current review is to specifically examine the overlap between the processing of value and/or valence with the processing of social stimuli and behaviors, and what these interactions can inform us about social cognition more broadly. As this question is fundamentally centered at the level of single neurons, this review will primarily focus on studies from the field of primate social neurophysiology [53].
2. Representations of valence and value in the primate amygdala
Early investigations of amygdala function, dating back to the classic study by Kluver and Bucy (1939), observed that temporal lobe lesions in rhesus monkeys produced a wide variety of heterogenous impairments. Deficits in learning stimulus-reinforcement associations [55,56] prompted subsequent questions if the primate amygdala represented positive or negative affective significance, also termed valence. At the time, this deficit was hypothesized to be a disruption in a visually based stimuli-reward associative pathway dependent on the amygdala. Anatomical projections from the visually-associated inferior temporal cortex to the amygdala [57–59] implicated the amygdala as a downstream recipient of a stimulus-recognition processes, hypothesized to support stimulus-reward association processes necessary for the evaluation of external stimuli in learning processes [56]. These foundational lesion studies of the amygdala eventually paved the way into far more sophisticated dissections of reinforcement learning using more selective lesions or behavioral measures [23–25,60–66] that are beyond the scope of this review.
Investigations of single neuron activity in the amygdala, through extracellular neurophysiological recordings, enabled researchers to examine how individual amygdala neurons in the primate brain alter their spiking responses to behavioral and perceptual manipulations. When discussing the selectivity of single neurons to stimulus or task parameters, it is important to consider the methods used in calculating selectively. Studies of stimulus-valence or stimulus-value associations easily lend themselves to comparisons of epochs aligned to differential stimulus presentations, e.g., examining the spiking rate of a neuron following presentation of a stimulus associated with a positively-valenced liquid rewards to a different stimulus associated with a negatively-valenced air puff. To derive a measure of selectivity, tied to the underlying contrast being made, the spiking activity of neurons is compared between these two conditions. In Figure 1, we have illustrated greatly simplified results in the form of single unit activity from selected single-unit recording experiments to highlight example encoding of various stimuli or task features. By examining these studies in more detail as we compare and contrast valence/value and social behavioral coding of amygdala neurons in the primate brain.
Figure 1.
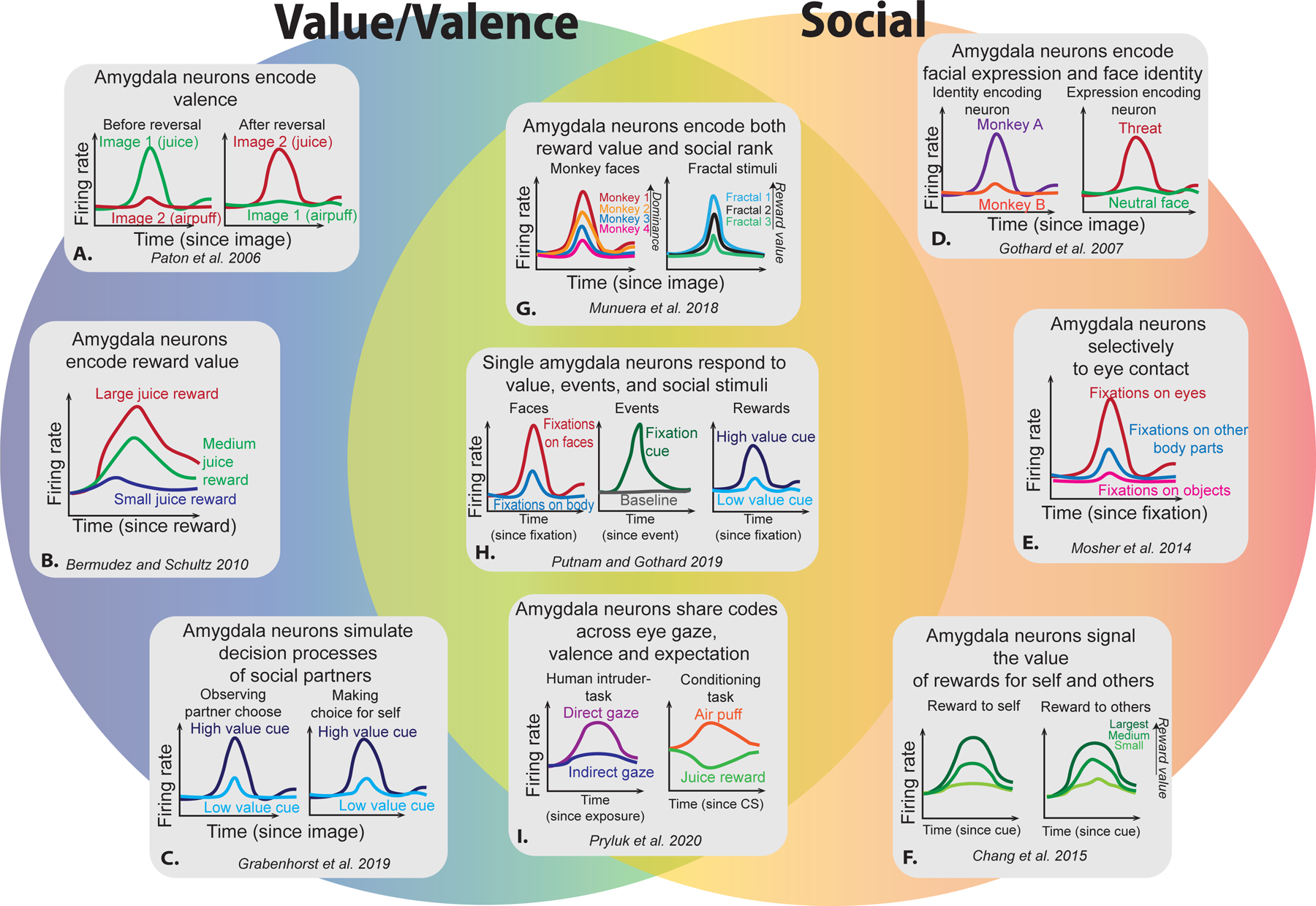
Illustrations of selected findings from amygdala studies examining value/ valence (left) and during social behaviors (right). These illustrations are based on the data from the indicated citations. Please see the text for full descriptions. A) Amygdala neurons selectively respond to stimuli that predict juice rewards, and maintained this valence encoding even after a reversal of valence for individual stimuli [15]. B) Amygdala neurons differentially represent large, medium, or small juice rewards [11]. C) Amygdala neurons signal value on both self and observational learning trials [67]. D) Neurons in the amygdala exhibit selectivity to facial identity or expression [36] as shown in images. E) Amygdala neurons selectivity respond to fixations on the eyes of conspecifics shown in videos [38]. F) Amygdala neurons signal rewards delivered to partner in a vicarious reward task [28]. G) Amygdala neurons hierarchically encode both the rank of conspecifics shown in images, and the reward values associated with images of fractals [14]. H) Single amygdala neurons exhibit selectivity across different domains, including facial selectivity, reward values associated with stimuli, and fixation cues [37]. Amygdala neurons use a similar coding schema across both gaze direction and expectation of aversive or appetitive stimuli [47].
Initial reports of single-neuron recordings from the primate amygdala indicated that not only did many neurons respond robustly to visual stimuli, but some of those did so in a manner selective for visual stimuli linked to rewards [35]. To specifically investigate if amygdala neurons were actually representing stimulus-affective associations, Nishijo and Nishino (1988) presented visual, auditory, or gustatory stimuli to monkeys that were linked to rewarding or aversive outcomes across these sensory domains. Out of a total of 585 single neurons sampled, the authors found that just over half of them were responsive in at least one of the domains, and a relatively large percentage (20%) were selective across sensory modalities. Notably, the authors identified that some amygdala neurons responded differentially between positive and negative stimuli, suggesting that the affective significance was indeed being signaled in the amygdala. It was not until later that these results were more thoroughly expanded upon by Paton and colleagues (2006), who presented images of fractal patterns as conditioned stimuli, associated with either positively-valenced liquid rewards or negatively-valenced air puffs. In their study, responses of single neurons recorded from the primate amygdala encoded the positive or negative valence associated with the stimuli presented, and individual neurons maintained this valence encoding even after a reversal of valence for individual stimuli (Fig. 1A). A follow-up study by Belova and colleagues (2007) examined the selectivity of primate amygdala neurons to positive or negative stimuli that were expected or unexpected, demonstrating that this additional dimension modulated the responses of different neuronal populations to either rewards or punishments, or to both [21]. The same authors later also showed that separate functional populations of neurons in the amygdala tracked positive or negative value for different states (fixation point, unconditioned stimulus, conditioned stimulus) during trace-conditioning [22]. Together, these studies demonstrate that amygdala neurons encode the valence of stimuli, and that often this valence information is modulated by expectation [21], time [22], or even spatial factors [16,17].
The coding of economic value, which is experimentally represented by the magnitude of rewards delivered to the subject, is a crucial variable in many theories of learning [69]. To examine if single neurons in the amygdala encoded the magnitude of reward value, Bermudez and Schultz (2010) presented a Pavlovian-conditioned visual stimulus to monkeys which predicted a varying sized liquid reward (equal chance of small, medium, and large rewards). Following the delivery of the liquid rewards, a subset (56/317, 18%) of the recorded amygdala neurons modulated their firing rates in accordance with the amount of liquid received, exhibiting parametric reward value coding (Fig. 1B). Although previous studies had performed similar tasks using stimuli that predicted either a large or small liquid reward, or air puff, to show that amygdala neurons differentiate between larger and smaller rewards [22], the use of at least three different reward values in this study clearly demonstrates a graded, parametric, response to reward magnitudes. A follow-up study by the same authors suggested that these signals may be encoding the specific expectations of reward, as amygdala neurons differentiated between rewards delivered instantaneously and those delivered at flat rates over a period of time [12]. Moreover, experiments in human patients undergoing neurosurgical treatments have extended these results, finding single neurons in the amygdala of awake patients that encode behaviorally assigned values of familiar foods (presented as pictures) [70]. Another study in human patients found valuation signals for pictures of foods that were irrespective of the task being performed, but only in the left amygdala [71]. A study by Leathers and Olson (2017) examined amygdala neural responses to cues predicting liquid rewards or air puffs that varied both in magnitude and valence. Their results found that that amygdala neurons, like in previous studies, differentiated large and small predicted rewards. Additionally, however, the authors varied the magnitude of air puffs and found that, unlike other brain regions [73], relatively few amygdala neurons encoded the magnitude of the air puffs [72]. These results overall provide evidence that amygdala neurons encode reward magnitude, and this information can be modulated by temporal sequence [12] and be linked to future plans to gain rewarding outcomes [74,75].
Interestingly, neurons in the primate amygdala do not merely represent value for oneself but also for other agents (Chang et al., 2015) and use this information to signal upcoming behaviors. For example, Grabenhorst and colleagues (2019) tested two monkeys in an observational learning task, where monkeys took turns choosing between stimuli associated with probabilistic rewards. Remarkably, a subset of neurons in the observer’s amygdala responded to high value stimuli when their partner was making choices and continued to track this value when it became their turn to choose between the same pair of stimuli [67] (illustrated in Fig. 1C), while another subset of neurons signaled a prediction of the partner’s choice during observational trials. These results demonstrate that the amygdala is tracking information about salient external stimuli, such as the magnitude of reward [11], the positive and negative affective significance [15], and more complex representations of value which are combined with information about space [17], time [12], and future plans [74,75].
3. Neural substrates of social behaviors in the primate amygdala
A role for the amygdala in shaping social behaviors was established early through observations of behavioral changes following amygdalectomies in rhesus monkeys [54,76,77]. The rich literature of lesion studies have often observed differential effects on social behaviors, which may depend on the age at which they are performed, and the social history or sex of the subject animals [52]. Although the body of evidence does not suggest that the amygdala is strictly necessary for any specific social behavior, it does indicate that the amygdala contribute and optimize to many different social behaviors [52]. This is corroborated by a wealth of studies utilizing functional magnetic resonance imaging in human subjects [78] that suggests the amygdala serves as a central “hub” in a network of brain regions supporting social function.
Results from single neurons recorded from the primate amygdala further substantiate the social function of the amygdala, which will be expanded upon below. In the examination of more naturalistic behaviors, such as studying social behaviors, the difficulties in determining what neuronal activity may truly represent are amplified. While some animal models of social behavior use a task that imposes a trial-like structure upon the subjects, such as presenting images or movies to subjects in discrete intervals [48,79], it is also important to examine behaviors under less constrained settings to avoid or minimize only studying forced operant behaviors. For example, in to to study naturally reinforced looking behaviors to the faces of conspecifics, subjects must be able to freely fixate on any part of a face or anywhere in the environment. Furthermore, any realistic stimuli presented to subjects, such as images or videos of conspecifics, may have priors or connotations unknown to human experimenters, possibly specific to that individual animal. For these reasons and others alike, recently there has been a move towards studying live interactions between two (or possible more) non-human primates [28,30,31,53,80–82,82,83]. With increasingly naturalistic experimental settings, however, comes an increased need to rule out possible alternative influences on neuronal activity through meticulous analyses, detailed observation of all behaviors ,and whenever possible additional peripheral physiological markers [27].
Neural substrates of face perception are found across several different brain regions, most notably in the temporal cortices [84–88], frontal cortices [89,90], and the amygdala [34,36,38,39,91,92]. Signals clustered in face patch regions in the superior temporal sulcus and inferotemporal cortex serve as the foundational processing modules for the ability to recognizes faces and facial features [86]. However, outside these more perceptual regions, the interpretation of face-specific signals becomes less straightforward. Faces, and particularly eyes, are incredibly powerful conveyors of social information [93], and it is not surprising that social gaze information is well represented in brain regions involved in social behaviors. For primates, faces and eyes have inherent value and are viewed preferentially starting from a young age [94–96]. Although this inherent saliency of faces and eyes likely biases neural processing towards face selectivity, other social information is also simultaneously encoded by amygdala neurons. The most clear example of this was shown in a study by Gothard and colleagues [36], where the authors presented pictures of conspecifics to monkeys while recording single neurons from the amygdala. They identified specific responses to facial identity, facial expression, or mixed responses that were selective for both facial identity and expression (Fig. 1D). These findings in amygdala neurons have been replicated in human patients undergoing neurosurgical recordings [34,91,92,97,98]. Given that eyes are the most salient features of the face, it is unsurprising that neural substrates of eye contacts are also found in the primate amygdala. Mosher and colleagues (2014) presented videos of conspecifics [48] to monkeys and examined the fixation evoked activity to looking at the eyes of the stimulus monkey (the conspecific monkey featured in the video) during periods when the stimulus monkey was looking at the camera or away from the camera mimicking direct or indirect eye contact, respectively. Neurons in the amygdala not only responded selectively (when compared to fixations on other parts of the face) to fixations at the eyes of the stimulus monkey; Fig. 1E), but a subpopulation of these neurons also differentially encoded fixations during the simulated mutual eye contact [38].
The primate amygdala also contains neuronal representations of non-visual social stimuli. Kuraoka and Nakamura (2007) presented monkeys either audiovisual (video clip with sound), visual (video with no sound), or auditory (only sound from the video) stimuli of conspecifics displaying emotional expressions and found that a majority of the neurons responded to at least one of the three emotional expressions (an aggressive threat, scream, and or complex coo calls). Most of these responses were to visual or audiovisual stimuli (77/79, 97%), but of those that responded to the audiovisual stimuli, 39 neurons (51%) showed a greater magnitude of the response to the audiovisual stimuli than to the visual stimuli alone [39]. This result, in addition to a population of neurons that responded to the auditory or visual component presented independently (20%), suggest that the primate amygdala processes social stimuli across sensory modalities. Indeed, even tactile and proprioceptive information is processed in the amygdala [99], which likely contributes to self-monitoring during the production of facial expressions [100]. The encoding of social stimuli across multiple sensory domains in the primate amygdala is partially explained by the multisensory responses exhibited by amygdala neurons, being responsive to combinations of visual, tactile, and auditory stimuli [101].
The social function of the amygdala extends beyond the perceptual level. Single neurons in the primate amygdala also represent core computations of social decision-making. Chang et al. (2015) employed a vicarious social reward task [81] where two monkeys are seated near to each other and one of those monkeys, designated as the ‘actor’, is able to make decisions that affect the reward outcome of themselves as well as a conspecific monkey. On some trials the actor chooses between making a prosocial choice by donating a liquid reward to the other monkey and an antisocial choice by donating the liquid reward to no one or an empty juice collection bottle. Using this paradigm, the authors had previously demonstrated that monkeys prefer to donate liquid to the other monkey over no one, a behavior supported by vicarious social reward [28,30,80,81,102]. By recording single unit activity from the amygdala of the actor monkey, the authors found that neurons signaled both the value of rewards delivered to the actor monkey and the conspecific monkey when the actor made active social decisions (but not on trials where the computer determined the identical outcomes) [28] (Fig. 1F). Crucially, the value tuning across all recorded neurons for the rewards to self and other was highly correlated, implicating that this “value-mirroring” signals may be part of a larger process in the amygdala signaling the emotional experience in empathy or theory of mind.
Collectively, the studies discussed in this section supports an intricate relationship between valence and social behavioral processing in the amygdala. The mechanism by which amygdala neurons enable this interaction remains an important avenue for research toward better understanding the function of the amygdala.
4. Intersections between value and social processing in the primate amygdala
Many brain structures are known for their involvement in multiple functions. In the case of the primate amygdala, neurons represent rewards and punishments as well as social stimuli. Indeed, the high level of anatomical connectivity of the amygdala with other brain regions supports this perspective on multifaceted functionality [78,103,104]. The amygdala is highly interconnected with the frontal cortices [105,106], sensory areas [103,107,108], and other subcortical structures [59,104,109–111], positioning it anatomically as a brain region that not only receives a wide breadth of information but also one which can affect behavior via cortical or sub-cortical projections [4,19,24,25,30,31,61,64,112]. Electrophysiological recordings from the amygdala can test if each of these functions is instantiated in the response properties of its component neurons. However, most studies to date, including the evidence presented above, almost exclusively examined the domains of valence/value processes and social processes separately. An important question then would be, do the neural specializations for valence/value processing overlap with neural specializations for social behavior? If these two roles are mediated entirely through the same populations of neurons, it might suggest that they are specific instantiations of a more general process. Alternatively, if the population of neurons that compute valence /value was completely segregated (either anatomically or functionally) from neurons that encoded social behaviors, it would imply that processing of these different dimensions has no impact on the other (at least within the amygdala). Logically, it is also possible for there to be a large degree of functional or anatomical overlap, allowing for a population of amygdala neurons that are sensitive to both social and value-related information with smaller proportions of neurons that are highly specialized to one of these functions. Indeed, this last schema, termed multidimensional processing [52], is supported by several recent studies. This perspective suggests that many single neurons in the amygdala are responsive to multiple, independent, dimensions of the environment. These dimensions likely extend beyond value and social information, given that single neurons in the amygdala have already been demonstrated to be multisensory [101]. In this section, we will focus in specifically on the overlap between valence/value and social behaviors to examine how this multidimensional processing may impact the processing of social behaviors in the amygdala.
Although the lens of multidimensional processing can be retrospectively applied to previous studies, one of the first attempts to directly examine this question was carried out by Munuera and colleagues (2018). In this study, the authors examined if there was a relationship between the coding of hierarchical rank of conspecific monkeys depicted in facial images and non-social images indicating varying amounts of juice rewards. By presenting images of familiar conspecifics within a known rank hierarchy, the authors demonstrated that amygdala neurons linearly encoded the hierarchical rank (Fig. 1G). The subjects were also shown fractals associated with zero, one, or two drops of liquid reward. By training a linear decoder on the reward-associated fractals but testing it with the spiking data from the trials where the subjects viewed facial images, the authors were able to demonstrate that same neuronal ensembles that encoded reward values were also encoding dominance hierarchies [14]. This result is particularly interesting because both the reward values and rank hierarchies have an inherent structure, which was shared in the coding schema used by the neurons for both the social and non-social stimuli. The authors also tested neurons recorded from the orbitofrontal cortex and anterior cingulate cortex but neither region strongly represented hierarchical rank.
Additionally, a recent study by Putnam and Gothard (2019) examined how single neurons in the amygdala encoded various dimensions of a complex choice task. In this task, monkeys were trained to associate the identity of conspecific monkeys or objects, shown in videos, with a fixed liquid reward for correct responses. As the monkey stimulus and object stimulus were presented in pairs in two simultaneously playing videos, the subject was able to freely view both videos and made a choice using experience from previous trials, after which he would receive the liquid reward associated with the stimulus monkey or object chosen. The authors aligned neural activity either to fixation cue proceeding the trial, to fixations on the stimuli themselves, or to fixations on regions of interest within the stimuli such as eyes or faces. By comparing neural activity across different behavioral epochs, the authors found that amygdala neurons encoded both non-social dimensions such as being responsive to task events or the value of the stimulus, but also social information such as differentiating between monkeys and objects or being selective for faces (Fig. 1H). Most importantly, many neurons in the amygdala were selective to more than one dimension of the task, and the number of neurons selective for all four task dimensions exceeded the level that would be expected by chance [37]. Whereas previous studies had already identified these selectivity dimensions in isolation [11,36,38,113], the results of this study argue that interpreting the selectivity of neuron without testing it across other stimuli or task parameters can be easily misleading. That is, labeling neurons with selectively modulated responses to faces or eye contacts within a specific experimental setting as strictly “face cells” or “eye cells”, respectively, is misleading outside that specific setting without also testing their selectivity for other dimensions across different stimuli or behaviors. Alternatively, conceptualizing these neurons as “face selective cells” or “eye selective cells”, where that selectivity is one of their possible functions is a more appropriate terminology.
Finally, to examine how amygdala neurons represented both valence and the gaze of others, Pryluk and colleagues (2020) compared neural activity between a human intruder test, where a partition was removed allowing the subject monkey to view a human experimenter, and a classical conditioning test, where the subject would receive either juice rewards or air puffs in all ten trials of a given block. Each human intruder test block consisted of multiple exposures to the human, who would pseudorandomly make direct eye contact with the subject monkey or avert their eyes. As has been demonstrated before, single neurons in the amygdala were responsive to both gaze (human intruder trials) and valence (classical conditioning trials) (Fig. 1I). Critically, however, a correlation of linear-regression coefficients for gaze and valence revealed a shared neural code between these two dimensions. Responses to air puffs were similar to direct gaze of the human intruder (theorized to also be aversive), while responses to juice rewards were in line with viewing the averted eye gaze of the human intruder [47]. Interestingly, unlike the amygdala neurons, neurons in the anterior cingulate cortex mostly represented valence, but did not represent gaze at the population level.
5. The social functions of the amygdala from the perspectives of computational, algorithmic, and implementational levels
The three studies in the previous section collectively demonstrate that processing of social stimuli and valence/value occurs in, at least partially, overlapping neural populations in the amygdala, supporting the concept of multidimensional processing. They also support that the processing of value and valence influences how social behaviors are processed in amygdala neurons. As a useful platform, we can examine these possible schemas in the framework of David Marr (1982), who established three levels of organization to examine information processing systems [114]. This approach has proven useful in examining related questions, such as the existence of a specifically “social brain” and has been explored in depth in a recent publication [51] (Fig. 2). Briefly, the most macroscopic level is computational, which represents the ultimate function of the information processing system. The logical operations which are applied to accomplish this function are done at the algorithmic level. Finally, there is the implementational level which is the physical instantiation of these algorithms.
Figure 2.
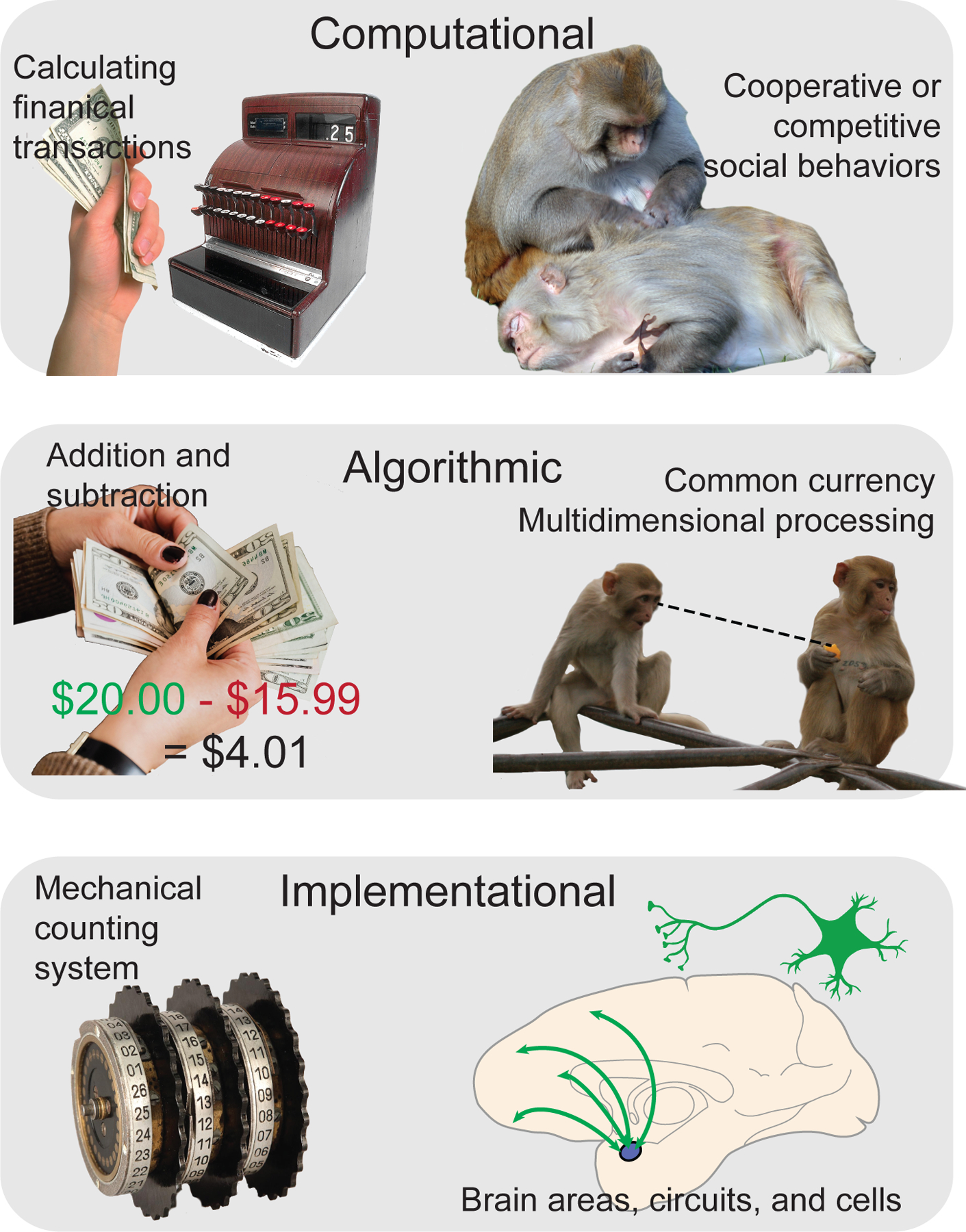
Levels of organization proposed by David Marr to understand information processing systems. This framework is adopted for social functions in this illustration [51]. The computational level (top) encompasses the ultimate goal of the process. For example, calculating financial transactions is the goal of a cash register (left) or engaging in cooperative social behaviors is the goal of certain brains areas. The algorithmic level (middle) is the logical processes by which this goal is accomplished. For a cash register to tally purchases, it must perform arithmetic (left), while social brain regions such as the amygdala may utilize a “common currency” coding schema, or multidimensional processing (right). Finally, these processes must be realized at the implementational level (bottom). In a cash register, the actual arithmetic is performed by a mechanical counting system (left), while different areas, circuits, or cells work together to process information (right).
At the broadest level, the computational goal of the amygdala is to correctly shape adaptative behaviors, which may specifically mean recognizing rewarding or aversive stimuli in the environment or being able to read social signals from, and send social signals to, conspecifics in order to engage in cooperative or competitive behaviors. Here, we propose two possible algorithms which could support these computational goals. The first is a “common currency” shared amongst social and non-social neural selectivity, which represents salient stimuli (both social and non-social) in a manner that is generalized. Two of the three aforementioned studies in the previous section exhibit results which could hypothetically support this view, as a shared neural code between gaze and valence [47] or between dominance and value [14] would be predicted by the common currency code in the amygdala. However, a theoretical drawback to the common currency framework is that it is less flexible across the many varied computational goals the brain must accomplish. An important point of consideration is that the use of a “common currency” schema amongst some neurons does not imply all neurons must code selectivity in this fashion. Moreover, although conceptualizing direct gaze as aversive may be ethologically valid in most behavioral contexts for rhesus monkeys (e.g., certainly from human intruders), it is not valid across all behavioral contexts [93,95,96,115]. For example, universally representing direct gaze as a negatively valenced signal does not explain the longer duration viewing of faces with direct gaze or eye contact in adult [96] and infant [95] rhesus monkeys compared to averted gaze. Monkeys will also forgo juice rewards to view the faces of high ranking conspecifics, even if those faces are presented with direct gaze [116]. Similarly, although linearly representing the value of juice rewards may fit well with neuroeconomic models, it may not apply equally to dominance hierarchies (especially for male rhesus monkeys) which are less stable in the wild than isolated laboratory housing [117]. An alternative algorithm could be coding schema where a large number of selectivity combinations are formed across the salient dimensions of stimuli or behaviors [52], in a manner that does not fall in line predictable constructs such value or valence. Such selectivity would be able to explain the greater number of neurons than expected by chance that are selective to multiple stimulus or task parameters by Putnam and Gothard (2019), although such multidimensionality was not evident in the study by Pryluk and colleagues (2020), whose results were more consistent with a common currency schema. Therefore, a hybrid, and more likely, schema is that multidimensional selectivity is an inherent property of amygdala neurons but learning and exposure may tend to drive some neural ensembles into using common coding schemas, such as the common currency schema, especially when those coding schemas neatly encapsulate the range of stimuli used in an experiment. To resolve these opposing theories, experiments with large populations of simultaneously recorded neurons and broad ranges of stimuli and behaviors would be necessary. Until recently, practical experimental limitations have prevented this. Now, however, advances in chronic recording methods in rhesus monkeys allow single-unit recordings from the same neurons over weeks and months to test the neurons under broad ranges of stimuli and behaviors [118–120]. Future experiments, utilizing such technological innovations would be able to test how multidimensionality and common currency schemas might or might not be related.
Finally, we propose two possible implementations which could explain the findings discussed in the previous section. The first is a serial implementation, where the amygdala is primarily computing the saliency of a stimulus while the accompanying context (e.g., valence, or direct vs. averted gaze) is provided by prefrontal regions. Alternatively, a parallel implementation would implicate both the amygdala and other brain regions computing various stimulus dimensions in parallel, where the communication or intermediary circuits between these regions serve to compare results. Under this latter model, it is possible that the amygdala may compute context more rapidly but less precisely than prefrontal regions such as the orbitofrontal cortex, and this tradeoff in speed may allow a rapid preparation for action [112,121] to be later be modified by, for example, the orbitofrontal cortical inputs. Given the prevalence of valence/value coding in the amygdala, a parallel implementation seems more likely. Temporary focal inactivation, through pharmacological means or designer receptors exclusively activated by designer drugs, of prefrontal cortices and the amygdala while recording from the other region would help test between these two hypotheses.
6. Concluding remarks
Here we have discussed how the primate amygdala contains neural specializations for both valence/value processing and social behaviors. Recent experiments have shown that these processes overlap at the level of single neurons in the amygdala, although the precise nature of this overlap still needs to be explored in more detail. Additionally, we have a poor understanding of the inter-nuclear differences between the structures that make up the amygdala, and how this contributes to the processing of social behaviors and valence/value. Although some studies have found differences in response properties and selectivity between the centromedial and basolateral [113] nuclei, there has yet to be a systematic electrophysiological probing of primate amygdala that integrates into the rich literature detailing the anatomical connections of the amygdala, and possibly reveals an organizational principle behind how the amygdala is representing multiple informational domains. Future experiments with advanced technology available today are able to shed light on the nature of the coupled processing of value and social behaviors in the amygdala, and these results may inform us about the function of the amygdala under a network-level perspective.
Acknowledgements
This work was supported by the National Institute of Mental Health (R01 MH120081, R01MH110750).
References
- [1].LeDoux J, Emotion Circuits in the Brain, Annu. Rev. Neurosci 23 (2000) 155–184. 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- [2].LeDoux J, The emotional brain, fear, and the amygdala, Cell. Mol. Neurobiol 23 (2003) 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rosen JB, The Neurobiology of Conditioned and Unconditioned Fear: A Neurobehavioral System Analysis of the Amygdala, Behav. Cogn. Neurosci. Rev 3 (2004) 23–41. 10.1177/1534582304265945. [DOI] [PubMed] [Google Scholar]
- [4].Duvarci S, Pare D, Amygdala Microcircuits Controlling Learned Fear, Neuron 82 (2014) 966–980. 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tye KM, Cone JJ, Schairer WW, Janak PH, Amygdala neural encoding of the absence of reward during extinction, J. Neurosci. Off. J. Soc. Neurosci 30 (2010) 116–125. 10.1523/JNEUROSCI.4240-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Morrison SE, Salzman CD, Re-valuing the amygdala, Curr. Opin. Neurobiol 20 (2010) 221–230. 10.1016/j.conb.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Murray EA, The amygdala, reward and emotion, Trends Cogn. Sci 11 (2007) 489–497. 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- [8].Baxter MG, Murray EA, The amygdala and reward, Nat. Rev. Neurosci 3 (2002) 563–573. 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- [9].Salzman CD, Paton JJ, Belova MA, Morrison SE, Flexible neural representations of value in the primate brain, Ann. N. Y. Acad. Sci 1121 (2007) 336–354. 10.1196/annals.1401.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].O’Neill P-K, Gore F, Salzman CD, Basolateral amygdala circuitry in positive and negative valence, Curr. Opin. Neurobiol 49 (2018) 175–183. 10.1016/j.conb.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bermudez MA, Schultz W, Reward magnitude coding in primate amygdala neurons, J. Neurophysiol 104 (2010) 3424–3432. 10.1152/jn.00540.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bermudez MA, Göbel C, Schultz W, Sensitivity to temporal reward structure in amygdala neurons, Curr. Biol. CB 22 (2012) 1839–1844. 10.1016/j.cub.2012.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Saez RA, Saez A, Paton JJ, Lau B, Salzman CD, Distinct Roles for the Amygdala and Orbitofrontal Cortex in Representing the Relative Amount of Expected Reward, Neuron 95 (2017) 70–77.e3. 10.1016/j.neuron.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Munuera J, Rigotti M, Salzman CD, Shared neural coding for social hierarchy and reward value in primate amygdala, Nat. Neurosci 21 (2018) 415–423. 10.1038/s41593-018-0082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Paton JJ, Belova MA, Morrison SE, Salzman CD, The primate amygdala represents the positive and negative value of visual stimuli during learning, Nature 439 (2006) 865–870. 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Peck CJ, Salzman CD, Amygdala neural activity reflects spatial attention towards stimuli promising reward or threatening punishment, ELife 3 (2014). 10.7554/eLife.04478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Peck CJ, Lau B, Salzman CD, The primate amygdala combines information about space and value, Nat. Neurosci 16 (2013) 340–348. 10.1038/nn.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Beyeler A, Namburi P, Glober GF, Simonnet C, Calhoon GG, Conyers GF, Luck R, Wildes CP, Tye KM, Divergent Routing of Positive and Negative Information from the Amygdala during Memory Retrieval, Neuron 90 (2016) 348–361. 10.1016/j.neuron.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Burgos-Robles A, Kimchi EY, Izadmehr EM, Porzenheim MJ, Ramos-Guasp WA, Nieh EH, Felix-Ortiz AC, Namburi P, Leppla CA, Presbrey KN, Anandalingam KK, Pagan-Rivera PA, Anahtar M, Beyeler A, Tye KM, Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment, Nat. Neurosci 20 (2017) 824–835. 10.1038/nn.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Beyeler A, Chang C-J, Silvestre M, Lévêque C, Namburi P, Wildes CP, Tye KM, Organization of Valence-Encoding and Projection-Defined Neurons in the Basolateral Amygdala, Cell Rep 22 (2018) 905–918. 10.1016/j.celrep.2017.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Belova MA, Paton JJ, Morrison SE, Salzman CD, Expectation modulates neural responses to pleasant and aversive stimuli in primate amygdala, Neuron 55 (2007) 970–984. 10.1016/j.neuron.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Belova MA, Paton JJ, Salzman CD, Moment-to-moment tracking of state value in the amygdala, J. Neurosci. Off. J. Soc. Neurosci 28 (2008) 10023–10030. 10.1523/JNEUROSCI.1400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Izquierdo A, Murray EA, Selective bilateral amygdala lesions in rhesus monkeys fail to disrupt object reversal learning, J. Neurosci. Off. J. Soc. Neurosci 27 (2007) 1054–1062. 10.1523/JNEUROSCI.3616-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rudebeck PH, Mitz AR, Chacko RV, Murray EA, Effects of amygdala lesions on reward-value coding in orbital and medial prefrontal cortex, Neuron 80 (2013) 1519–1531. 10.1016/j.neuron.2013.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rudebeck PH, Ripple JA, Mitz AR, Averbeck BB, Murray EA, Amygdala Contributions to Stimulus-Reward Encoding in the Macaque Medial and Orbital Frontal Cortex during Learning, J. Neurosci. Off. J. Soc. Neurosci 37 (2017) 2186–2202. 10.1523/JNEUROSCI.0933-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Murray EA, Wise SP, Interactions between orbital prefrontal cortex and amygdala: advanced cognition, learned responses and instinctive behaviors, Curr. Opin. Neurobiol 20 (2010) 212–220. 10.1016/j.conb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gothard KM, Mosher CP, Zimmerman PE, Putnam PT, Morrow JK, Fuglevand AJ, New perspectives on the neurophysiology of primate amygdala emerging from the study of naturalistic social behaviors, Wiley Interdiscip. Rev. Cogn. Sci 9 (2017) e1449. 10.1002/wcs.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chang SWC, Fagan NA, Toda K, Utevsky AV, Pearson JM, Platt ML, Neural mechanisms of social decision-making in the primate amygdala, Proc. Natl. Acad. Sci. U. S. A 112 (2015) 16012–16017. 10.1073/pnas.1514761112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rutishauser U, Mamelak AN, Adolphs R, The primate amygdala in social perception - insights from electrophysiological recordings and stimulation, Trends Neurosci 38 (2015) 295–306. 10.1016/j.tins.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dal Monte O, Chu CCJ, Fagan NA, Chang SWC, Specialized medial prefrontal–amygdala coordination in other-regarding decision preference, Nat. Neurosci 23 (2020) 565–574. 10.1038/s41593-020-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gangopadhyay P, Chawla M, Dal Monte O, Chang SWC, Prefrontal-amygdala circuits in social decision-making, Nat. Neurosci 24 (2021) 5–18. 10.1038/s41593-020-00738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Adolphs R, Tranel D, Damasio H, Damasio A, Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala, Nature 372 (1994) 669–672. 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- [33].Spezio ML, Huang P-YS, Castelli F, Adolphs R, Amygdala Damage Impairs Eye Contact During Conversations with Real People, J. Neurosci 27 (2007) 3994–3997. 10.1523/JNEUROSCI.3789-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Minxha J, Mosher C, Morrow JK, Mamelak AN, Adolphs R, Gothard KM, Rutishauser U, Fixations gate species-specific responses to free viewing of faces in the human and macaque amygdala, Cell Rep 18 (2017) 878–891. 10.1016/j.celrep.2016.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sanghera MK, Rolls ET, Roper-Hall A, Visual responses of neurons in the dorsolateral amygdala of the alert monkey, Exp. Neurol 63 (1979) 610–626. [DOI] [PubMed] [Google Scholar]
- [36].Gothard KM, Battaglia FP, Erickson CA, Spitler KM, Amaral DG, Neural responses to facial expression and face identity in the monkey amygdala, J. Neurophysiol 97 (2007) 1671–1683. 10.1152/jn.00714.2006. [DOI] [PubMed] [Google Scholar]
- [37].Putnam PT, Gothard KM, Multidimensional Neural Selectivity in the Primate Amygdala, ENeuro 6 (2019). 10.1523/ENEURO.0153-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mosher CP, Zimmerman PE, Gothard KM, Neurons in the monkey amygdala detect eye contact during naturalistic social interactions, Curr. Biol. CB 24 (2014) 2459–2464. 10.1016/j.cub.2014.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kuraoka K, Nakamura K, Responses of single neurons in monkey amygdala to facial and vocal emotions, J. Neurophysiol 97 (2007) 1379–1387. 10.1152/jn.00464.2006. [DOI] [PubMed] [Google Scholar]
- [40].Adolphs R, The Social Brain: Neural Basis of Social Knowledge, Annu. Rev. Psychol 60 (2009) 693–716. 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mobbs D, Adolphs R, Fanselow MS, Barrett LF, LeDoux JE, Ressler K, Tye KM, Viewpoints: Approaches to defining and investigating fear, Nat. Neurosci 22 (2019) 1205–1216. 10.1038/s41593-019-0456-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Paré D, Quirk GJ, When scientific paradigms lead to tunnel vision: lessons from the study of fear, Npj Sci. Learn 2 (2017) 1–8. 10.1038/s41539-017-0007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schoenbaum G, Takahashi Y, Liu T-L, McDannald MA, Does the orbitofrontal cortex signal value?, Ann. N. Y. Acad. Sci 1239 (2011) 87–99. 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Stalnaker TA, Cooch NK, Schoenbaum G, What the orbitofrontal cortex does not do, Nat. Neurosci 18 (2015) 620–627. 10.1038/nn.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhou J, Gardner MPH, Schoenbaum G, Is the core function of orbitofrontal cortex to signal values or make predictions?, (2020). 10.31234/osf.io/nzjm9. [DOI] [PMC free article] [PubMed]
- [46].Hayden B, Niv Y, The case against economic values in the brain, (2020). 10.31234/osf.io/7hgup. [DOI] [PubMed]
- [47].Pryluk R, Shohat Y, Morozov A, Friedman D, Taub AH, Paz R, Shared yet dissociable neural codes across eye gaze, valence and expectation, Nature 586 (2020) 95–100. 10.1038/s41586-020-2740-8. [DOI] [PubMed] [Google Scholar]
- [48].Mosher CP, Zimmerman PE, Gothard KM, Videos of conspecifics elicit interactive looking patterns and facial expressions in monkeys, Behav. Neurosci 125 (2011) 639–652. 10.1037/a0024264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Davis M, Whalen PJ, The amygdala: vigilance and emotion, Mol. Psychiatry 6 (2001) 13–34. [DOI] [PubMed] [Google Scholar]
- [50].Brothers L, The neural basis of primate social communication, Motiv. Emot 14 (1990) 81–91. 10.1007/BF00991637. [DOI] [Google Scholar]
- [51].Lockwood PL, Apps MAJ, Chang SWC, Is There a ‘Social’ Brain? Implementations and Algorithms, Trends Cogn. Sci 24 (2020) 802–813. 10.1016/j.tics.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gothard KM, Multidimensional processing in the amygdala, Nat. Rev. Neurosci 21 (2020) 565–575. 10.1038/s41583-020-0350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chang SWC, An Emerging Field of Primate Social Neurophysiology: Current Developments, ENeuro 4 (2017). 10.1523/ENEURO.0295-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Klüver H, Bucy PC, Preliminary analysis of functions of the temporal lobes in monkeys, Arch. Neurol. Psychiatry 42 (1939) 979–1000. 10.1001/archneurpsyc.1939.02270240017001. [DOI] [PubMed] [Google Scholar]
- [55].Jones B, Mishkin M, Limbic lesions and the problem of stimulus—Reinforcement associations, Exp. Neurol 36 (1972) 362–377. 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- [56].Spiegler BJ, Mishkin M, Evidence for the sequential participation of inferior temporal cortex and amygdala in the acquisition of stimulus-reward associations, Behav. Brain Res 3 (1981) 303–317. 10.1016/0166-4328(81)90002-4. [DOI] [PubMed] [Google Scholar]
- [57].Herzog AG, Van Hoesen GW, Temporal neocortical afferent connections to the amygdala in the rhesus monkey, Brain Res 115 (1976) 57–69. 10.1016/0006-8993(76)90822-2. [DOI] [PubMed] [Google Scholar]
- [58].Jones EG, Powell TP, An anatomical study of converging sensory pathways within the cerebral cortex of the monkey, Brain J. Neurol 93 (1970) 793–820. 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- [59].Nauta WJ, Whitlock DG, Subcortical projections from the temporal neocortex in Macaca mulatta, J. Comp. Neurol 106 (1956) 183–212. 10.1002/cne.901060107. [DOI] [PubMed] [Google Scholar]
- [60].Costa VD, Dal Monte O, Lucas DR, Murray EA, Averbeck BB, Amygdala and Ventral Striatum Make Distinct Contributions to Reinforcement Learning, Neuron 92 (2016) 505–517. 10.1016/j.neuron.2016.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fiuzat EC, Rhodes SEV, Murray EA, The Role of Orbitofrontal-Amygdala Interactions in Updating Action-Outcome Valuations in Macaques, J. Neurosci. Off. J. Soc. Neurosci 37 (2017) 2463–2470. 10.1523/JNEUROSCI.1839-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Izquierdo A, Murray EA, Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys, J. Neurophysiol 91 (2004) 2023–2039. 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- [63].Rhodes SEV, Charles DP, Howland EJ, Murray EA, Amygdala lesions in rhesus monkeys fail to disrupt object choices based on internal context, Behav. Neurosci 126 (2012) 270–278. 10.1037/a0027229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rhodes SEV, Murray EA, Differential effects of amygdala, orbital prefrontal cortex, and prelimbic cortex lesions on goal-directed behavior in rhesus macaques, J. Neurosci. Off. J. Soc. Neurosci 33 (2013) 3380–3389. 10.1523/JNEUROSCI.4374-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Taswell CA, Costa VD, Basile BM, Pujara MS, Jones B, Manem N, Murray EA, Averbeck BB, Effects of Amygdala Lesions on Object-Based Versus Action-Based Learning in Macaques, Cereb. Cortex N. Y. N 1991. (2020). 10.1093/cercor/bhaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Vaidya AR, Pujara MS, Petrides M, Murray EA, Fellows LK, Lesion Studies in Contemporary Neuroscience, Trends Cogn. Sci 23 (2019) 653–671. 10.1016/j.tics.2019.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Grabenhorst F, Báez-Mendoza R, Genest W, Deco G, Schultz W, Primate Amygdala Neurons Simulate Decision Processes of Social Partners, Cell 177 (2019) 986–998.e15. 10.1016/j.cell.2019.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Nishijo H, Ono T, Nishino H, Single neuron responses in amygdala of alert monkey during complex sensory stimulation with affective significance, J. Neurosci. Off. J. Soc. Neurosci 8 (1988) 3570–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Niv Y, Reinforcement learning in the brain, J. Math. Psychol 53 (2009) 139–154. 10.1016/j.jmp.2008.12.005. [DOI] [Google Scholar]
- [70].Jenison RL, Rangel A, Oya H, Kawasaki H, Howard MA, Value Encoding in Single Neurons in the Human Amygdala during Decision Making, J. Neurosci 31 (2011) 331–338. 10.1523/JNEUROSCI.4461-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Mormann F, Bausch M, Knieling S, Fried I, Neurons in the Human Left Amygdala Automatically Encode Subjective Value Irrespective of Task, Cereb. Cortex 29 (2019) 265–272. 10.1093/cercor/bhx330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Leathers ML, Olson CR, In monkeys making value-based decisions, amygdala neurons are sensitive to cue value as distinct from cue salience, J. Neurophysiol 117 (2017) 1499–1511. 10.1152/jn.00564.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Leathers ML, Olson CR, In Monkeys Making Value-Based Decisions, LIP Neurons Encode Cue Salience and Not Action Value, Science 338 (2012) 132–135. 10.1126/science.1226405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Grabenhorst F, Hernádi I, Schultz W, Prediction of economic choice by primate amygdala neurons, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 18950–18955. 10.1073/pnas.1212706109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hernádi I, Grabenhorst F, Schultz W, Planning activity for internally generated reward goals in monkey amygdala neurons, Nat. Neurosci 18 (2015) 461–469. 10.1038/nn.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Weiskrantz L, Behavioral changes associated with ablation of the amygdaloid complex in monkeys, J. Comp. Physiol. Psychol 49 (1956) 381–391. 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- [77].Rosvold HE, Mirsky AF, Pribram KH, Influence of amygdalectomy on social behavior in monkeys, J. Comp. Physiol. Psychol 47 (1954) 173–178. 10.1037/h0058870. [DOI] [PubMed] [Google Scholar]
- [78].Bickart KC, Dickerson BC, Barrett LF, The amygdala as a hub in brain networks that support social life, Neuropsychologia 63 (2014) 235–248. 10.1016/j.neuropsychologia.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].McMahon DBT, Russ BE, Elnaiem HD, Kurnikova AI, Leopold DA, Single-unit activity during natural vision: diversity, consistency, and spatial sensitivity among AF face patch neurons, J. Neurosci. Off. J. Soc. Neurosci 35 (2015) 5537–5548. 10.1523/JNEUROSCI.3825-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chang SWC, Coordinate transformation approach to social interactions, Front. Neurosci 7 (2013) 147. 10.3389/fnins.2013.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Chang SWC, Winecoff AA, Platt ML, Vicarious Reinforcement in Rhesus Macaques (Macaca Mulatta), Front. Neurosci 5 (2011). 10.3389/fnins.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Haroush K, Williams ZM, Neuronal Prediction of Opponent’s Behavior during Cooperative Social Interchange in Primates, Cell 160 (2015) 1233–1245. 10.1016/j.cell.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Fan S, Dal Monte O, Chang SWC, Levels of naturalism in social neuroscience research, IScience (in submission) (n.d.). [DOI] [PMC free article] [PubMed]
- [84].Tsao DY, Livingstone MS, Mechanisms of face perception, Annu. Rev. Neurosci 31 (2008) 411–437. 10.1146/annurev.neuro.30.051606.094238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Tsao DY, Freiwald WA, Tootell RBH, Livingstone MS, A cortical region consisting entirely of face-selective cells, Science 311 (2006) 670–674. 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Chang L, Tsao DY, The Code for Facial Identity in the Primate Brain, Cell 169 (2017) 1013–1028.e14. 10.1016/j.cell.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Freiwald WA, Tsao DY, Livingstone MS, A face feature space in the macaque temporal lobe, Nat. Neurosci 12 (2009) 1187–1196. 10.1038/nn.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Desimone R, Albright TD, Gross CG, Bruce C, Stimulus-selective properties of inferior temporal neurons in the macaque, J. Neurosci 4 (1984) 2051–2062. 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tsao DY, Schweers N, Moeller S, Freiwald WA, Patches of face-selective cortex in the macaque frontal lobe, Nat. Neurosci 11 (2008) 877–879. 10.1038/nn.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Barat E, Wirth S, Duhamel J-R, Face cells in orbitofrontal cortex represent social categories, Proc. Natl. Acad. Sci. U. S. A 115 (2018) E11158–E11167. 10.1073/pnas.1806165115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Rutishauser U, Tudusciuc O, Neumann D, Mamelak AN, Heller AC, Ross IB, Philpott L, Sutherling WW, Adolphs R, Single-unit responses selective for whole faces in the human amygdala, Curr. Biol. CB 21 (2011) 1654–1660. 10.1016/j.cub.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Mormann F, Niediek J, Tudusciuc O, Quesada CM, Coenen VA, Elger CE, Adolphs R, Neurons in the human amygdala encode face identity, but not gaze direction, Nat. Neurosci 18 (2015) 1568–1570. 10.1038/nn.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Emery NJ, The eyes have it: the neuroethology, function and evolution of social gaze, Neurosci. Biobehav. Rev 24 (2000) 581–604. [DOI] [PubMed] [Google Scholar]
- [94].Frank MC, Vul E, Johnson SP, Development of infants’ attention to faces during the first year, Cognition 110 (2009) 160–170. 10.1016/j.cognition.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Simpson EA, Paukner A, Pedersen EJ, Ferrari PF, Parr LA, Visual preferences for direct gaze faces in infant macaques (Macaca mulatta) with limited face exposure, Dev. Psychobiol 61 (2019) 228–238. 10.1002/dev.21797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Leonard TK, Blumenthal G, Gothard KM, Hoffman KL, How macaques view familiarity and gaze in conspecific faces, Behav. Neurosci 126 (2012) 781–791. 10.1037/a0030348. [DOI] [PubMed] [Google Scholar]
- [97].Cao R, Wang J, Lin C, Rutishauser U, Todorov A, Li X, Brandmeir N, Wang S, Feature-based encoding of face identity by single neurons in the human medial temporal lobe, BioRxiv (2020) 2020.09.01.278283. 10.1101/2020.09.01.278283. [DOI]
- [98].Wang S, Yu R, Tyszka JM, Zhen S, Kovach C, Sun S, Huang Y, Hurlemann R, Ross IB, Chung JM, Mamelak AN, Adolphs R, Rutishauser U, The human amygdala parametrically encodes the intensity of specific facial emotions and their categorical ambiguity, Nat. Commun 8 (2017) 14821. 10.1038/ncomms14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Mosher CP, Zimmerman PE, Fuglevand AJ, Gothard KM, Tactile Stimulation of the Face and the Production of Facial Expressions Activate Neurons in the Primate Amygdala, ENeuro 3 (2016). 10.1523/ENEURO.0182-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Livneh U, Resnik J, Shohat Y, Paz R, Self-monitoring of social facial expressions in the primate amygdala and cingulate cortex, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 18956–18961. 10.1073/pnas.1207662109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Morrow J, Mosher C, Gothard K, Multisensory neurons in the primate amygdala, J. Neurosci. Off. J. Soc. Neurosci (2019). 10.1523/JNEUROSCI.2903-18.2019. [DOI] [PMC free article] [PubMed]
- [102].Chang SWC, Gariépy J-F, Platt ML, Neuronal reference frames for social decisions in primate frontal cortex, Nat. Neurosci 16 (2013) 243–250. 10.1038/nn.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Freese JL, Amaral DG, The Synaptic Organization of Projections from the Amygdala to Visual Cortical Areas TE and V1 in the Macaque Monkey, J. Comp. Neurol 496 (2006) 655–667. 10.1002/cne.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Price JL, Amaral DG, An autoradiographic study of the projections of the central nucleus of the monkey amygdala, J. Neurosci. Off. J. Soc. Neurosci 1 (1981) 1242–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Ghashghaei HT, Hilgetag CC, Barbas H, Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala, NeuroImage 34 (2007) 905–923. 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Carmichael ST, Price JL, Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys, J. Comp. Neurol 363 (1995) 615–641. 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- [107].Freese JL, Amaral DG, The organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey, J. Comp. Neurol 486 (2005) 295–317. 10.1002/cne.20520. [DOI] [PubMed] [Google Scholar]
- [108].Amaral DG, Insausti R, Retrograde transport of D-[3H]-aspartate injected into the monkey amygdaloid complex, Exp. Brain Res 88 (1992) 375–388. 10.1007/BF02259113. [DOI] [PubMed] [Google Scholar]
- [109].Friedman DP, Aggleton JP, Saunders RC, Comparison of hippocampal, amygdala, and perirhinal projections to the nucleus accumbens: combined anterograde and retrograde tracing study in the Macaque brain, J. Comp. Neurol 450 (2002) 345–365. 10.1002/cne.10336. [DOI] [PubMed] [Google Scholar]
- [110].Nagai T, Kimura H, Maeda T, McGeer PL, Peng F, McGeer EG, Cholinergic projections from the basal forebrain of rat to the amygdala, J. Neurosci. Off. J. Soc. Neurosci 2 (1982) 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Woolf NJ, Butcher LL, Cholinergic projections to the basolateral amygdala: a combined Evans Blue and acetylcholinesterase analysis, Brain Res. Bull 8 (1982) 751–763. [DOI] [PubMed] [Google Scholar]
- [112].Pascoe JP, Kapp BS, Electrophysiological characteristics of amygdaloid central nucleus neurons during Pavlovian fear conditioning in the rabbit, Behav. Brain Res 16 (1985) 117–133. [DOI] [PubMed] [Google Scholar]
- [113].Mosher CP, Zimmerman PE, Gothard KM, Response characteristics of basolateral and centromedial neurons in the primate amygdala, J. Neurosci. Off. J. Soc. Neurosci 30 (2010) 16197–16207. 10.1523/JNEUROSCI.3225-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Marr D, Vision: A computational investigation into the human representation and processing of visual information, henry holt and co, Inc N. Y. NY 2 (1982). [Google Scholar]
- [115].Emery NJ, Lorincz EN, Perrett DI, Oram MW, Baker CI, Gaze following and joint attention in rhesus monkeys (Macaca mulatta), J. Comp. Psychol. Wash. DC 1983 111 (1997) 286–293. [DOI] [PubMed] [Google Scholar]
- [116].Deaner RO, Khera AV, Platt ML, Monkeys Pay Per View: Adaptive Valuation of Social Images by Rhesus Macaques, Curr. Biol 15 (2005) 543–548. 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- [117].Vessey SH, Dominance among Rhesus Monkeys, Polit. Psychol 5 (1984) 623–628. 10.2307/3791232. [DOI] [Google Scholar]
- [118].Bondar IV, Leopold DA, Richmond BJ, Victor JD, Logothetis NK, Long-Term Stability of Visual Pattern Selective Responses of Monkey Temporal Lobe Neurons, PLOS ONE 4 (2009) e8222. 10.1371/journal.pone.0008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].McMahon DBT, Bondar IV, Afuwape OAT, Ide DC, Leopold DA, One month in the life of a neuron: longitudinal single-unit electrophysiology in the monkey visual system, J. Neurophysiol 112 (2014) 1748–1762. 10.1152/jn.00052.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].McMahon DBT, Jones AP, Bondar IV, Leopold DA, Face-selective neurons maintain consistent visual responses across months, Proc. Natl. Acad. Sci. U. S. A 111 (2014) 8251–8256. 10.1073/pnas.1318331111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Applegate CD, Frysinger RC, Kapp BS, Gallagher M, Multiple unit activity recorded from amygdala central nucleus during Pavlovian heart rate conditioning in rabbit, Brain Res 238 (1982) 457–462. [DOI] [PubMed] [Google Scholar]


