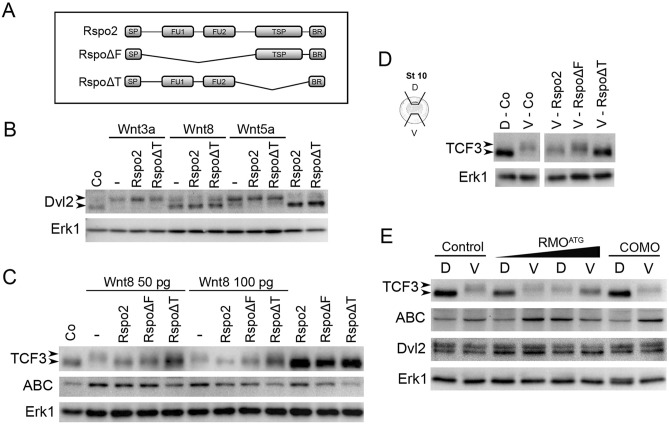
Figure 4.
Rspo2 inhibits TCF3 phosphorylation. (A) Schematic of Rspo2 deletion constructs. SP, signal peptide; FU1, furin-like domain 1; FU2, furin-like domain 2; TSP, thrombospondin type 1 domain; BR, the basic amino acid-rich domain. (B,C) Effects of Rspo2 constructs on Wnt-dependent Dvl2 phosphorylation (B) and TCF3 phosphorylation and β-catenin levels (C). Four-cell stage embryos were injected animally with Wnt8 DNA (50 pg or 100 pg) or Wnt8, Wnt3a or Wnt5a RNAs (1 ng each) and Rspo2, Rspo∆F or Rspo∆T RNAs (0.5 ng each) as indicated. Ectoderm explants were dissected at stage 9 and cultured until stage 12 for immunoblotting with antibodies against Dvl2, TCF3, ABC (non-phosphorylated β-catenin). Arrowheads indicate the position of phosphorylated (upshifted) and non-phosphorylated Dvl2 or TCF3 proteins. Erk1 controls for loading. (D) Effects of Rspo2 constructs (0.5 ng each) on TCF3 phosphorylated by endogenous signals. Dorsal marginal zone (D) and ventral marginal zone (V) were dissected from the control and injected embryos at stage 10 and cultured until stage 12.5 for immunoblotting with anti-TCF3 antibodies as shown. Control D and V groups were run in the same gel but separately from the other groups (see Supplementary Fig. 5). (E) Effects of Rspo2 depletion on TCF3 phosphorylation by endogenous signals. DMZ and VMZ explants of embryos injected with control MO (COMO, 20 ng) or RMOATG (20 ng) were dissected and analyzed by immunoblotting as in (B,C).