FIGURE 1.
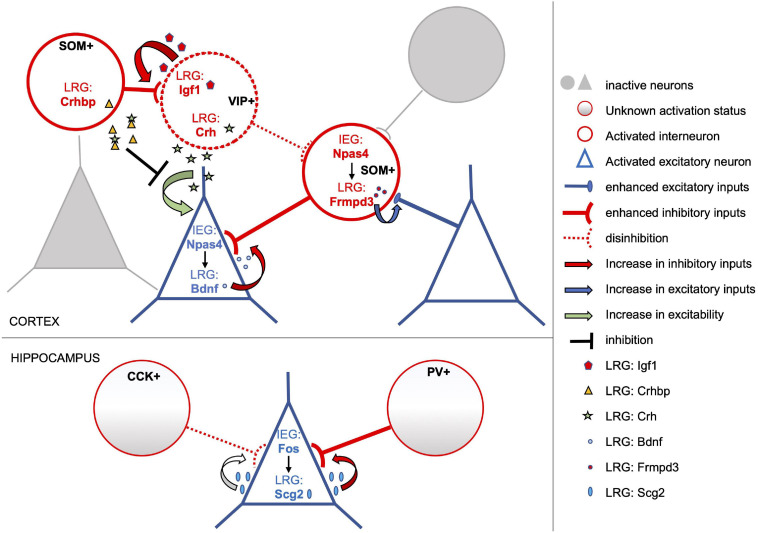
Subtype-specific activity-dependent transcriptional programs shape circuit rearrangements in response to experience. The model depicts examples of activity-induced LRGs which modulate connectivity and/or excitability of distinct neuronal subtypes, overall enhancing the inhibitory tone onto activated pyramidal neurons. Excitatory and inhibitory neurons of the activated neuronal ensemble are depicted as blue triangles and red circles, respectively. In cortical excitatory neurons the IEG Npas4 drives expression of several LRGs including Bdnf (blue dots), which in turn promote an increase in the number of inhibitory inputs onto these cells. Conversely, Npas4 expression in activated SOM + inhibitory neurons triggers a transcriptional program that includes the LRG Frmpd3 (red dots), which induces an increase in excitation onto the GABAergic cell (Spiegel et al., 2014). Activity-dependent transcriptional profiles of cortical VIP + interneurons include the LRGs Igf1 (red pentagons) and Crh (green stars). Secreted Igf1 promotes inhibition of VIP + neurons (Mardinly et al., 2016), which often operate by disinhibiting principal neurons. On the other hand, VIP + neurons also release Crh which can increase the excitability of nearby pyramidal cells. However, this effect may be tampered by Crhbp (yellow triangles), an inhibitor of Chr secreted by SOM + neurons in response to stimulation (Hrvatin et al., 2018). Overall, activity-dependent transcriptional responses of both excitatory and inhibitory cortical neurons appear to converge in increasing the inhibitory tone onto activated excitatory neurons. Similarly, in the hippocampus, Fos expression in activated excitatory neurons induces the LRG Scg2, a precursor to four neuropeptides, which increases perisomatic inhibition by PV-interneurons albeit weakening that of CCK-interneurons (Yap et al., 2021).