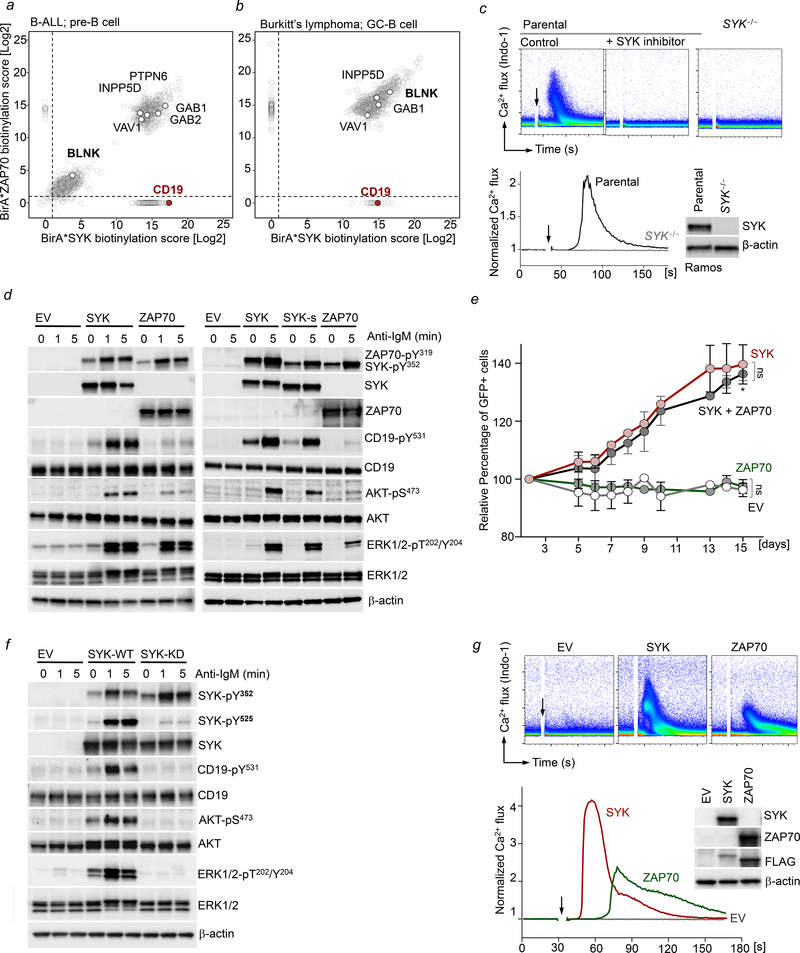
Figure 2: Interactome analyses of SYK and ZAP70 defines kinase-specific integration into B-cell networks.
BioID-interactome analysis of SYK and ZAP70 in Kasumi-2 B-ALL cells (a) and in Ramos lymphoma cells (b). Biotinylated peptide abundance ratios of SYK and ZAP70 BirA*-expressing cells are illustrated. (c) Single-cell clone SYK−/− derived Ramos cells were generated using RNP CRISPR mediated targeting of SYK. BCR-induced Ca2+ flux following IgM stimulation (10 mg/mL) was measured in parental Ramos cells +/− pre-treatment with the SYK inhibitor PRT062607 (5 μmol/L) and in Ramos SYK−/− cells using Indo-1. (d) SYK−/− Ramos cells were reconstituted with MSCV-IRES-GFP empty vector (EV), SYK (SYK or SYK-s) or ZAP70 constructs and analyzed for phosphorylation of BCR-activated substrates by WB. (e) SYK−/− Ramos cells reconstituted with EV, SYK, ZAP70 or SYK+ZAP70 were mixed 1:1 with untransduced SYK−/− cells, and changes in GFP were measured by flow cytometry over 15 days. Data are shown as mean ± standard error of mean (SEM) of two independent experiments. Statistical significance for day 15 was calculated using an unpaired Student’s t-test (*p<0.05). (f) Ramos SYK−/− cells were reconstituted with EV, SYK-wildtype (WT) or SYK-kinase dead (KD) constructs and analyzed for phosphorylation of BCR-activated substrates. (g) BCR-induced Ca2+ flux following IgM stimulation (10 μg/mL) was measured in SYK−/− Ramos cells reconstituted with EV, SYK or ZAP70 using Indo-1. Levels of SYK and ZAP70 were measured by Western blotting using antigen-specific antibodies, and a FLAG-antibody against each tagged protein (inset).