Abstract
Visceral artery pseudoaneurysms are rare and potentially fatal unless recognized and treated immediately. Here we present to our mind the first documented case of a ruptured pseudoaneurysm involving the left gastric artery giving rise to Michels’ Type II replaced left hepatic artery. An 84-year-old female presented with an acute rupture of such an aneurysm post radical cystectomy. CT Angiogram prior to intervention was key for appropriate catheter selection. Endovascular embolization proved effective, and the patient recovered unremarkably. The case report includes a brief discussion regarding the investigation and management of such ruptures, as well as the rarity of the variant anatomy described.
Keywords: Interventional radiology, Vascular Anatomy, Variant Anatomy, Gastrointestinal, Pseudoanaeurysm, Endovascular Management, Aneurysm
Case report
Informed consent was acquired from the patient discussed in this case report. Institutional review board approval was not required for the publication of this case.
An 84-year-old Caucasian female was admitted electively for radical cystectomy, hysterectomy, pelvic dissection, & ileal conduit insertion for the treatment of urothelial carcinoma in situ. The patient's medical history included hiatus hernia, gastro-esophageal reflux disease, hypothyroidism, appendectomy, and surgical removal of an ovarian cyst. The patient had no history of trauma or infection, nor any history of atherosclerosis or vasculitis.
On day one post-operatively full blood examination revealed a hemoglobin of 77g/L, reduced from a preoperative baseline of 130g/L (110-160). The patient was otherwise well, received two units of packed red blood cells and was stepped down from intensive care to the ward on day 2 postoperatively.
On day 3 postoperatively the patient developed constipation, nausea, bilious vomiting, and significant hematemesis. The patient's abdomen was found to be distended but soft and nontender. Air entry was reduced at the left lung base and the patient was supported with 4L oxygen via Hudson mask, maintaining an oxygen saturation of 93%. A same-day abdominal CT scan was acquired for further investigation.
Preoperative abdominal CT 3 months prior to her cystectomy had demonstrated the large hiatus hernia. On this CT the left gastric and left hepatic arteries were normal in caliber and appearance (Fig. 1).
Fig. 1.
CT axial demonstrating normal left gastric artery pre-cystectomy.
The postoperative abdominal CT scan demonstrated a 50 × 120 × 100 mm posterior mediastinal hematoma displacing the large hiatus hernia. There was dilation of the left gastric artery extending 10 cm in length. The maximal diameter of this dilated segment was 15 mm. This segment gave rise to the left hepatic artery which supplied most of the left lobe of the liver (Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6).
Fig. 2.
Pseudoaneurysm of the left gastric post-cystectomy.
Fig. 3.
CT sagittal of large hiatus hernia and posterior mediastinal hematoma. Arrow: pseudoaneurysm of left gastric artery.
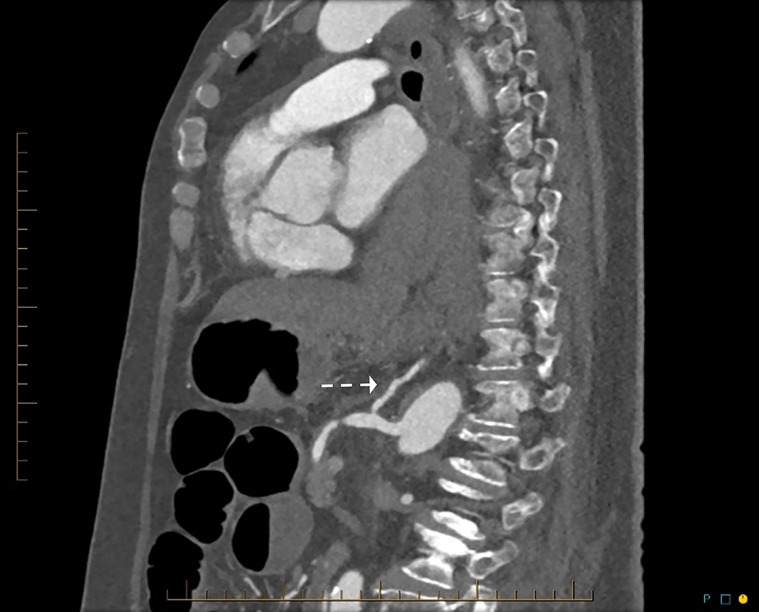
Fig. 4.
CT sagittal of large hiatus hernia and posterior mediastinal hematoma. Dashed arrow: vertical origin of the left gastric artery, allowing easy cannulation using reverse-curve catheter.
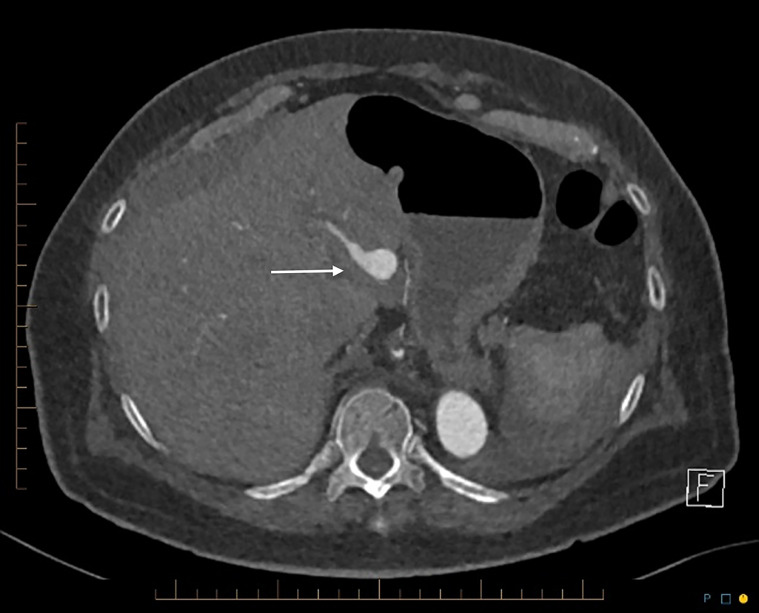
Fig. 5.
CT axial demonstrates pseudoaneurysm of replaced left hepatic artery arising from left gastric artery.
Fig. 6.
CT sagittal demonstrates pseudoaneurysm of replaced left hepatic artery arising from left gastric artery.
There was some suspicion of a possible fistula between the left gastric and/or hepatic and left portal vein on this CT scan that was not demonstrated on subsequent angiogram. There was no evidence of altered perfusion of the liver or stomach. The aorta and arteries demonstrated no significant atherosclerosis. There was evidence of the recent cystectomy and a postoperative ileus. There was no evidence of iatrogenic injury. The patient was transferred that same day to a tertiary center for management under interventional radiology.
On the night of day 3 postoperatively the endovascular procedure was performed under general anesthetic. The CT angiogram demonstrated early vertical branching of the left gastric artery 1.5 cm from the ostium of the coeliac trunk (Fig. 4). It was therefore thought to be best selected using a 5Fr Simmons 1 catheter (Cordis, Miami Lakes, Florida) with a 6Fr guide sheath (Cook Inc., Bloomington, Indianapolis).
The intra-procedural angiogram demonstrated an aneurysmal dilation of the distal left gastric artery, and both the left extra and intrahepatic arteries (Fig. 7). There was no evidence of the previously suspected communication with the left portal vein. The segment II and III intrahepatic branches enhanced normally. Inflow and outflow embolization was performed. The left intrahepatic artery was embolized using 0.018” pushable Nester and Tornado coils (Cook Inc., Bloomington, Indianapolis) through a 0.021” Direxion microcatheter (Boston Scientific, Marlborough, Massachusetts). The short left gastric artery feeding into the proximal pseudoaneurysm was also embolized using pushable 0.018” coils. Inter-lock detachable coils (Boston Scientific, Marlborough, Massachusetts) were then used to embolize the proximal left hepatic artery (Fig. 8).
Fig. 7.
Mesenteric angiogram: aneurysmal dilatation of the distal left gastric artery and replaced left hepatic artery.
Fig. 8.
Upstream and downstream embolization of the replaced left hepatic artery and distal left gastric artery.
Post intervention the patient was admitted to the high-dependency unit for 24-hours. The post intervention hemoglobin (Hb) on day one was noted at 84, whilst liver biochemistry was mildly deranged: ALT 128H U/L (<34), AST 147H U/L (<31) & LD 305H U/L (120-250). All observations were stable, and the patient was stepped down to the surgical ward on day 2. Recovery was unremarkable and the patient was discharged home on day 7 post intervention. At 6-weeks post discharge liver biochemistry had returned to the normal range, and Hb had returned to 130g/L.
Discussion
In the most common anatomy, trifurcation of the coeliac axis gives rise to the splenic, left gastric (LGA) and common hepatic arteries (CHA) [1]. CHA then divides distally into the right (RHA) and left (LHA) hepatic branches. In 1966 Michels identified ten variants to the most common hepatic arterial anatomy. Variant hepatic arterial anatomy has been shown to occur in at least 20% of individuals, with several variations described [2]. Michels’ work represents the most commonly cited classification system, as it distinguishes between a replaced and accessory LHA [1].
The Michels Type II variant describes a replaced LHA arising from the LGA. This variant has been estimated to occur in 3% of the population and is the form identified in this case report [2]. A database search (MEDLINE, EMBASE, Scopus) revealed just one case report of a ruptured true aneurysm demonstrating this variant anatomy that was treated endovascularly, and one that was managed with open surgical approach [3], [5]. We believe this is the first documented case describing rupture of a LGA pseudoaneurysm giving rise to, and including, Michels Type II replaced LHA. Radical cystectomy prior to rupture is also previously undocumented.
True visceral artery aneurysm (VAA) requires distinction from visceral artery pseudoaneurysm (VAPA). In VAA dilation involves the entirety of the vascular wall: tunica intima, media and adventitia, and may be sacciform or fusiform in morphology [4]. In VAPA an enclosed hematoma communicates with the arterial lumen between the tunica media and adventitia [4]. VAA has been associated with atherosclerosis, fibromuscular dysplasia and vasculitis [3,4]. VAPA is most often encountered in the setting of trauma, infection or iatrogenic insult during hepatobiliary procedures [3,4]. Radical cystectomy prior to rupture, as demonstrated in this case, is most unusual and defies plausible anatomic explanation. VAPA involving the LGA is particularly rare. A single center analysis considering all presenting VAA and VAPA over ten years identified 253 cases, of which two (0.8%) were pseudoaneurysms of the LGA [8].
A majority of VAPA are discovered as a result of their rupture, which carries a significant mortality risk reported between 25 and 70% [7]. VAPAs carry a risk of rupture reported up to 76.3%, vs a reported 3.1% for VAA [8]. Pseudoaneurysms may rupture intra and/or extrahepatically. Extrahepatic rupture may cause intraperitoneal hemorrhage, whilst intrahepatic rupture may hemorrhage into the biliary tree and result in Quincke's triad of biliary colic, gastrointestinal bleeding and obstructive jaundice [10]. This case describes extrahepatic rupture with posterior mediastinal hemorrhage as a result of the significant hiatus hernia. Clinically, organ dysfunction and hemorrhagic signs are predominant in the case of rupture [11].
Diagnostic assessment may be made via 3 modalities: ultrasound, CT, and CTA/DSA [6]. Unruptured aneurysms may present on ultrasound as hypoechoic lesions, with the classic “yin-yang” bidirectional flow sign demonstrated on Doppler when the dilatation is sufficiently large [6]. VAPA are challenging to identify on ultrasound, particularly when surrounded by a hematoma. On contrast CT an unruptured VAPA will demonstrate enhancement with Hounsfield units concordant with aortic attenuation, whereas acute hemorrhage in the setting of rupture will demonstrate the high-density blush of extravasation [6]. CTA/DSA represent the gold standard in diagnosis due to their ability to thoroughly evaluate and localize the aneurysm without the need for exploratory laparotomy [6]. In this case CTA represented an essential tool for procedural planning and correct instrument selection. This expedited the procedure itself, reducing the need for trial and error with catheter selection and resulting in significantly reduced intra-procedural radiation exposure.
The essential premise of management is exclusion of the aneurysm from circulation by either surgical or endovascular approach. The dual nature of the hepatic blood supply, being 70% supplied by the portal vein and 30% by the hepatic arteries, is important to note given possible approaches to management [12]. Surgical management approaches include open aneurysmectomy and end-to-end anastomosis, with or without aortohepatic bypass [13]. Depending upon the collateral blood supply to the hepatic parenchyma, ligation may also be employed in more straight-forward cases, otherwise a concomitant resection of the involved portion of the liver may be required [9].
Endovascular management is minimally invasive and characterized by a shorter postinterventional recovery period, however its provision is limited by logistics and the availability of requisite facilities. The endovascular approach is driven by several factors including aneurysm size, location and morphology. Occlusion of the parent vessel may be achieved via the so-called “sandwich” technique of inserting upstream and downstream injectable coils or plugs, a technique employed to good effect in this case [4]. Alternately, the aneurysm may be packed, and thrombin injected percutaneously [4]. Finally, a covered stent may be implanted, though this carries a risk of stent occlusion and thus is disadvantaged where arteries are insufficiently wide [13].
A systematic review and meta-analysis of VAA and VAPA management demonstrated low short and long-term mortality post intervention, without significant differences between either surgical or endovascular approaches [14]. The same study suggested that an open surgical approach was accompanied by numerous and greater rates of complication, potentially justifying the endovascular approach as a first choice [14]. The rarity of the condition results in small sample sizes for such studies.
Assessment of hepatic biomarkers has been found valuable in evaluating both post-procedural liver function and its subsequent recovery [15]. A mild LFT derangement due to moderate interruption of hepatic arterial supply post procedure is to be expected, and recovery to a normal reference range at follow-up, as demonstrated in this case, represents a good outcome.
Conclusion / learning points
-
•
VAPA are rare and potentially fatal unless recognized and treated immediately.
-
•
This is the first documented case of combined gastric artery and/or replaced left hepatic artery pseudoaneurysm.
-
•
Endovascular intervention performed by interventional radiology demonstrates fewer post-procedural complications than does an open approach and proved effective and expedient in managing the rupture presented here.
-
•
CTA prior to procedure is an essential component of planning and instrument selection, particularly in the extraordinary anatomical circumstances documented in this case.
Footnotes
Acknowledgments: None.
Competing Interests: None.
Material not presented at an SIR Annual Scientific Meeting.
Reference
- 1.Ye Z., Ye S., Zhou D., Zheng S., Wang W. A rare variation of celiac trunk and hepatic artery complicating pancreaticoduodenectomy: a case report and literature review. Med. 2017;96:pe8969. doi: 10.1097/MD.0000000000008969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noussios G., Dimitriou I., Chatzis I., Katsourakis A. The main anatomic variations of the hepatic artery and their importance in surgical practice: review of the literature. J Clin Med Res. 2017;9(4):248–252. doi: 10.14740/jocmr2902w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch J., Montgomery A., Shelmerdine S., Taylor J. Case report: ruptured aneurysm of an aberrant left hepatic artery. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-201409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiaradia M., Novelli L., Deux J., Tacher V., Mayer J., You K. Ruptured visceral artery aneurysms. Diagnostic and Interventional Imaging. 2015;96:797–806. doi: 10.1016/j.diii.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Altaca G. Ruptured aneurysm of replaced left hepatic artery as a cause of haemorrhagic shock: a challenge of diagnosis and treatment. Interactive Cardiovasc and Thoracic Surg. 2012;14(2):220–222. doi: 10.1093/icvts/ivr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valluru B. Significance of radiology in the diagnosis and management of ruptured left gastric artery aneurysm associated with acute pancreatitis. Medicine. 2019;98(10):e14825. doi: 10.1097/MD.0000000000014824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdelgabar A., d'Archambeau O., Maes J., Van den Brande F., Cools P., Rutsaert R. Visceral artery pseudoaneurysm: two case reports and a review of the literature. J Med Case Rep. 2017;11(126) doi: 10.1186/s13256-017-1291-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitton M., Dappa E., Jungmann F., Kloeckner R., Schotten S., Wirth G. Visceral artery aneurysms: incidence, management, and outcome analysis in a tertiary care center over one decade. Euro Radiol. 2015;25(7):2004–2014. doi: 10.1007/s00330-015-3599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pulli R., Dorigo W., Troisi N., Pratesi G., Innocenti A., Pratesi C. Surgical treatment of visceral artery aneurysms: a 25-year experience. J Vascular Surg. 2008;48(2):334–342. doi: 10.1016/j.jvs.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 10.Belfonte C., Sanderson A., Dejenie F. Quincke's triad: a rare complication of a common outpatient procedure: 734. Am J Gastroenterol. 2011;106:S277. [Google Scholar]
- 11.Juntermanns B., Bernheim J., Karaindros K., Walensi M., Hoffman J. Visceral artery aneurysms. Gefässchirurgie. 2018;23(S1):19–22. doi: 10.1007/s00772-018-0384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rush N., Sun H., Nakanishi Y., Mneimneh W., Kwo P., Saxena R. Hepatic arterial buffer response: pathologic evidence in non-cirrhotic human liver with extrahepatic portal vein thrombosis. Modern Pathol. 2016;28(5):489–499. doi: 10.1038/modpathol.2016.43. [DOI] [PubMed] [Google Scholar]
- 13.Marone E., Mascia D., Kahlberg A., Chiara B., Tshomba Y., Chiesa R. Is open repair still the gold standard in visceral artery aneurysm management. Ann Vasc Surg. 2011;25(7):936–946. doi: 10.1016/j.avsg.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Barrionuevo P., Malas M., Nejim B., Haddad A., Morrow A., Ponce O. A systematic review and meta-analysis of the management of visceral artery aneurysms. J Vasc Surg. 2019;70(5):1694–1699. doi: 10.1016/j.jvs.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 15.Jaunoo S., Tang T., Uzoigwe C., Walsh S., Gaunt M. Hepatic artery aneurysm repair: a case report. J Med Case Rep. 2009;3(1):18. doi: 10.1186/1752-1947-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]