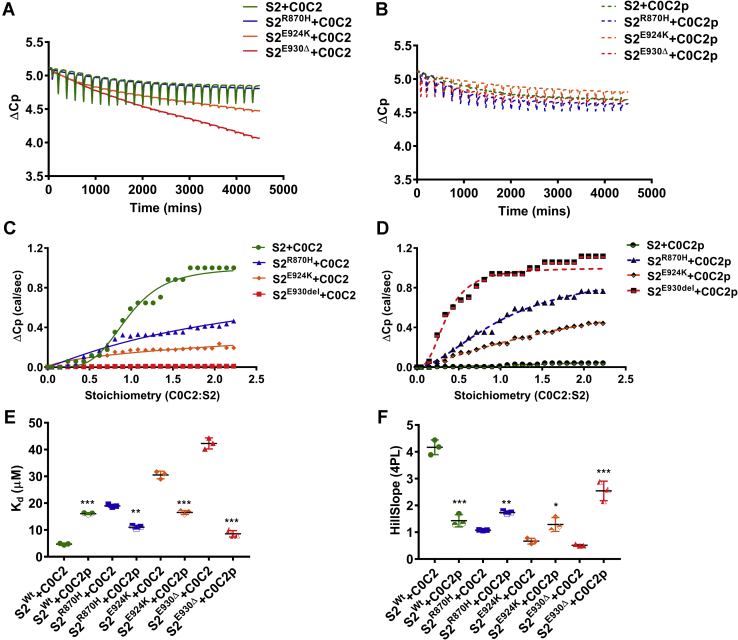
Figure 4.
Mutant hS2 displays a higher rate of heat change upon binding to phosphorylated hC0-C2 protein by ITC assay.A, representative traces for change in heat observed upon titrating hS2Wt (green), hS2R870H (blue), hS2E924K (orange), and E930Δ (red) proteins to hC0-C2 proteins. B, representative traces for change in heat observed upon titrating hS2Wt (green dashed), hS2R870H (blue dashed), hS2E924K (orange dashed), and hS2E930Δ (red dashed) proteins to hC0-C2p proteins. C, sigmoidal curve for titration of hS2Wt to hC0-C2 yields the dissociation constant (1/slope) and stoichiometry of the reaction. Titration of hS2Wt (green), hS2R870H (blue), hS2E924K (orange), and hS2E930Δ (red) proteins against dephosphorylated hC0-C2 proteins. D, sigmoidal curve for titration of hS2Wt (green dashed), hS2R870H (blue dashed), hS2E924K (orange dashed), and hS2E930Δ (red dashed) proteins to phosphorylated hC0-C2 protein. E, change in affinity for hS2Wt to hC0-C2 (solid) and hC0-C2p (half solid) proteins. F, change in slope for hS2Wt proteins to hC0-C2 (solid) and hC0-C2p (half solids) protein calculated by four-parameter logistic curve. Statistical analyses were performed by one-way ANOVA with Tukey’s multiple comparison test with single pooled variance. n = 3, where each n value was performed for hC0-C2 and hC0-C2p to minimize variance. Data are expressed as the mean ± SEM. See Table 3 for analysis of main factors and interactions. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001 versus hS2Wt to hC0-C2 control. hS2, human cardiac myosin S2; hS2E924K, human recombinant proximal S2 protein with E924K mutation; hS2E930Δ, human recombinant proximal S2 protein with E930Δ mutation; hS2R870H, human recombinant proximal S2 protein with R870H mutation; hS2Wt, human recombinant proximal S2 WT protein; ITC, isothermal titrating calorimetry; S2, subfragment 2 region.