Abstract
Background and Aims
We compared risk of acute liver injury and mortality in patients with COVID‐19 and current, past, and no HBV infection.
Approach and Results
This was a territory‐wide retrospective cohort study in Hong Kong. Patients with COVID‐19 between January 23, 2020, and January 1, 2021, were identified. Patients with hepatitis C or no HBsAg results were excluded. The primary outcome was mortality. Acute liver injury was defined as alanine aminotransferase or aspartate aminotransferase ≥2 × upper limit of normal (ULN; i.e., 80 U/L), with total bilirubin ≥2 × ULN (i.e., 2.2 mg/dL) and/or international normalized ratio ≥1.7. Of 5,639 patients included, 353 (6.3%) and 359 (6.4%) had current and past HBV infection, respectively. Compared to patients without known HBV exposure, current HBV‐infected patients were older and more likely to have cirrhosis. Past HBV‐infected patients were the oldest, and more had diabetes and cardiovascular disease. At a median follow‐up of 14 (9‐20) days, 138 (2.4%) patients died; acute liver injury occurred in 58 (1.2%), 8 (2.3%), and 11 (3.1%) patients with no, current, and past HBV infection, respectively. Acute liver injury (adjusted HR [aHR], 2.45; 95% CI, 1.52‐3.96; P < 0.001), but not current (aHR, 1.29; 95% CI, 0.61‐2.70; P = 0.507) or past (aHR, 0.90; 95% CI, 0.56‐1.46; P = 0.681) HBV infection, was associated with mortality. Use of corticosteroid, antifungal, ribavirin, or lopinavir–ritonavir (adjusted OR [aOR], 2.55‐5.63), but not current (aOR, 1.93; 95% CI, 0.88‐4.24; P = 0.102) or past (aOR, 1.25; 95% CI, 0.62‐2.55; P = 0.533) HBV infection, was associated with acute liver injury.
Conclusion
Current or past HBV infections were not associated with more liver injury and mortality in COVID‐19.
Abbreviations
- aHR
adjusted HR
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- ANCOVA
analysis of covariance
- anti‐HBc
hepatitis B core antibody
- anti‐HBs
hepatitis B surface antibody
- aOR
adjusted OR
- ASMD
absolute standardized mean difference
- AST
aspartate aminotransferase
- CDARS
Clinical Data Analysis and Reporting System
- CHB
chronic hepatitis B
- DM
diabetes mellitus
- GBM
generalized boosted model
- ICD‐9‐CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IPTW
inverse probability of treatment weighting
- IQR
interquartile range
- NA
nucleos(t)ide analogue
- PS
propensity score
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- ULN
upper limit of normal
COVID‐19 has spread rapidly throughout the world since late 2019. It has resulted in more than 129 million confirmed cases and 2.83 million deaths globally as of April 3, 2021.( 1 ) Liver injury is commonly observed in patients with COVID‐19, in the form of either hepatitis or cholestasis or both,( 2 ) and has been shown to be associated with adverse clinical outcomes.( 3 , 4 , 5 ) This has raised concerns over whether COVID‐19 would lead to worse clinical outcomes in patients with chronic liver diseases. It is of particular concern in the Asia‐Pacific region as almost 7% of the estimated 5 billion people living in this region have chronic HBV (CHB) infection.( 6 )
There is evolving evidence demonstrating the impact of HBV infection on patients with COVID‐19. A report described the clinical characteristics of 105 patients with COVID‐19 who also suffered from CHB, in whom liver injury, which occurred in 27.6% of patients, was associated with mortality.( 7 ) Yet how much of such liver injury was contributed by CHB was not addressed in this study as patients who did not have CHB but did have COVID‐19 were not included as a control group. Interestingly, a cohort of 571 patients with COVID‐19 in which 15 had HBV infection suggested that HBV‐infected patients experienced fewer adverse clinical outcomes.( 8 ) However, other studies with 20, 50, and 134 patients with COVID‐19 and HBV infection, respectively, did not show any difference in clinical outcomes.( 5 , 9 , 10 )
Another major concern is the interaction of various therapeutic options for COVID‐19 with HBV and its antiviral treatment. Immunomodulators, particularly potent corticosteroids such as dexamethasone,( 11 ) are now recommended as the treatment option in patients with severe COVID‐19. Corticosteroid is well known to cause potentially fatal HBV reactivation and hepatitis flare,( 12 ) even in patients with past exposure to HBV.( 13 ) Furthermore, the hepatotoxicity of other COVID‐19 therapeutics in patients with HBV infection has not been adequately evaluated. In this territory‐wide cohort study in Hong Kong, we aimed to compare the incidence of liver injury and mortality in patients with COVID‐19 with current, past, and no HBV infections. We also described their serial liver biochemistries, with special focus on the prognostic role of current and past HBV infection and liver injury.
Materials and Methods
Study Design and Data Source
We performed a territory‐wide retrospective cohort study using data from the Clinical Data Analysis and Reporting System (CDARS) under the management of the Hospital Authority, the sole public health care provider in Hong Kong.( 3 ) CDARS is an electronic health care database that contains patients’ demographic, diagnostic, procedural, and drug prescription and dispensing history, as well as laboratory results of all public hospitals and clinics in Hong Kong.( 14 ) All clinical information in CDARS is anonymized to ensure confidentiality. The International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) coding was adopted in CDARS; its use to identify medical conditions has been found to be 99% accurate when referenced to clinical, laboratory, imaging, and endoscopic results from the electronic medical records.( 15 ) Territory‐wide epidemiological studies of various infectious diseases were previously conducted using CDARS.( 3 , 16 , 17 , 18 ) All suspected and confirmed cases of COVID‐19 in Hong Kong are reported to the Department of Health and hospitalized under the care of the Hospital Authority. We performed testing for severe acute respiratory syndrome–coronavirus 2 (SARS‐CoV‐2) using RT‐PCR for both symptomatic patients presenting to outpatient clinics and hospitals as well as asymptomatic close contacts of confirmed patients and inbound travelers.
Subjects
Consecutive patients with COVID‐19 from January 23, 2020, to January 1, 2021, were identified by virological results (Supplementary Table S1). Patients with unavailable HBsAg results and HCV infection were excluded. Patients with current HBV infection were defined by HBsAg positivity and/or by ICD‐9‐CM diagnosis codes and/or by use of antiviral treatment for CHB. Past HBV infection was defined by negative HBsAg with positive hepatitis B core antibody (anti‐HBc) and/or positive hepatitis B surface antibody (anti‐HBs) if the patient was born before the launch of the universal neonatal vaccination program in Hong Kong, i.e., before 1988. We report the clinical characteristics of the identified patients with COVID‐19 and compared the patients with COVID‐19 and current, past, and no HBV infections. Patients were followed until death, discharge, last follow‐up date (January 20, 2021), or up to 60 days of follow‐up, whichever came first. Details on clinical evaluation and management of the patients are described in the Supporting Information. The study protocol was approved by the Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee (ref. no. 2020.074). The requirement for informed consent was waived by the institutional review committee due to the retrospective nature of the study.
Data collection
Data were retrieved from CDARS on January 22, 2021. Baseline date was defined as the date of diagnosis of COVID‐19 by virological results. Demographic data including date of birth and sex were captured. Death and its date were captured and ascertained using data from CDARS and the Hong Kong Death Registry. At baseline, hematological and virological parameters, liver and renal biochemistries, and other relevant laboratory parameters were collected. Thereafter, serial laboratory parameters, as well as SARS‐CoV‐2 viral assays were collected until the last follow‐up date (Supporting Table S1). We also retrieved data on other relevant diagnoses, procedures, concomitant drugs, and exposure to antivirals, antibiotics and antifungals, corticosteroids, interferon‐beta, immunoglobulin, and other COVID‐19 therapeutics before baseline and during hospitalization (Supporting Table S2).
Definitions
The primary endpoint was all‐cause mortality. Acute liver injury was defined as alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) ≥ 2 × upper limit of normal (ULN), with total bilirubin ≥2 × ULN and/or international normalized ratio ≥1.7.( 3 ) ULNs of ALT and AST were defined according to the criteria of the Asian Pacific Association for the Study of the Liver (40 U/L for both genders).( 19 ) ULN of total bilirubin was defined as 19 µmol/L (i.e., 1.1 mg/mL). ULN of alkaline phosphatase (ALP) was defined by each of the local laboratories based on age and gender. ULN of gamma‐glutamyl transferase was 40 U/L.
HCV infection was defined based on viral serology, use of antiviral treatment, and/or ICD‐9‐CM diagnosis codes.( 17 ) Liver‐related outcomes were defined based on ICD‐9‐CM diagnosis and procedure codes (Supporting Table S3). Significant comorbidities were defined as follows: hypertension was identified by any use of antihypertensive drugs and/or ICD‐9‐CM diagnosis codes; diabetes mellitus (DM) was defined by exposure to any antidiabetic agents and/or hemoglobin A1c ≥ 6.5% and/or fasting plasma glucose ≥7 mmol/L and/or the ICD‐9‐CM diagnosis codes for DM (250.00‐250.93).( 20 ) Liver cirrhosis was identified by ICD‐9‐CM diagnosis codes for cirrhosis and its related complications and/or platelet counts <100 × 109/L in a measurement at least 30 days before COVID‐19 diagnosis (Supporting Table S3). Other comorbidities were defined by ICD‐9‐CM diagnosis codes and/or medications (Supporting Table S3).
Statistical analysis
Data were analyzed using SPSS (version 25.0; SPSS, Inc., Chicago, IL), R software (4.0.3; R Foundation for Statistical Computing, Vienna, Austria), and SAS (9.4; SAS Institute Inc., Cary, NC). Continuous variables were expressed as mean ± SD or median (interquartile range [IQR]), as appropriate, while categorical variables were presented as frequency (percentage). Qualitative and quantitative differences between groups were analyzed by chi‐squared test or Fisher’s exact tests for categorical parameters and Student t test, Mann‐Whitney U test, one‐way ANOVA, or Kruskal‐Wallis test for continuous parameters, as appropriate. Median ALT level before and after HBV antiviral treatment and corticosteroid therapy between groups was compared by Quade’s analysis of covariance (ANCOVA).
Propensity score (PS), the conditional probability of having current HBV infection, was estimated among three groups of patients with current, past, and no HBV infections, to control for confounders and reduce selection bias.( 21 , 22 ) Twenty‐one clinical characteristics were included in the PS. We developed PS by generalized boosted models (GBMs) to capture nonlinear effects and interaction terms. GBM has been shown to provide less prediction error and more stable weights than logistic regression.( 23 , 24 , 25 ) The four stopping rules, namely the mean and maximum of the absolute standardized mean difference (ASMD) and of the Kolmogorov‐Smirnov statistic, were adopted to determine the optimal iteration of GBM. The stopping rule with overall the best balance of clinical characteristics and effective sample size was selected.( 26 ) In the inverse probability of treatment weighting (IPTW) analysis, we applied average treatment effect on the treated weighting, so the baseline clinical characteristics of patients with past or no HBV infection had nearly identical distributions after IPTW to those with current HBV infection.( 25 ) The balance of baseline clinical characteristics between patients was assessed by ASMD; an ASMD < 0.2 indicated a good balance.( 23 , 27 )
Before PS estimation, missing data were assumed missing at random and replaced by multiple imputation by chained equations to create 20 complete data sets after 10 initial burn‐in iterations.( 28 , 29 ) The imputed baseline variables (missing percentage) were serum creatinine (0.1%), albumin (0.1%), ALT (0.1%), total bilirubin (0.1%), ALP (0.1%), lactate dehydrogenase (1.1%), C‐reactive protein (1.0%), hemoglobin (0.04%), white cell counts (0.04%), lymphocyte (0.4%), neutrophil (0.4%), and platelet (0.04%). The variables included in the imputation models were all covariates included in PS estimation, occurrence of mortality, and the corresponding Nelson‐Aalen estimator of the cumulative hazard at the time of event or censoring.( 30 ) All imputed values were constrained within plausible ranges.
HRs and adjusted HRs (aHRs) with 95% CIs of current or past HBV infection referenced to no HBV infection on the primary endpoint were estimated by Cox proportional hazards regression. We adjusted for patients’ demographic, presence of acute liver injury, liver cirrhosis, comorbidities, and other relevant laboratory parameters, as shown in Supporting Table S4; backward stepwise elimination was performed to select statistically significant covariates. Weighted Cox proportional hazards regression was used in PS weighting and matching analysis. Clinical characteristics with ASMD ≥ 0.2 after PS balancing were adjusted in the weighted Cox model for double robustness. Robust (empirical) variance estimates were obtained to calculate 95% CIs.( 31 ) The overall coefficient estimates and standard errors were computed by combining the estimates obtained on each individual multiple imputation data set using Rubin’s rules.( 32 ) Schoenfeld residual plots were used to assess the proportional hazards assumption, which did not detect any significant violations.
ORs and adjusted ORs (aORs) with 95% CIs for acute liver injury were estimated by logistic regression. We included the following covariates: HBV exposure (current, past, or no HBV infection), age, gender, presence of cirrhosis and DM, and use of corticosteroids, remdesivir, interferon‐beta, ribavirin, lopinavir‐ritonavir, antibiotics, or antifungals during hospitalization; none were excluded in this analysis due to missing data. Significant covariates were selected by backward stepwise elimination. Goodness of fit was assessed by the Hosmer‐Lemeshow goodness‐of‐fit test. All statistical tests were two‐sided. Statistical significance was taken as P < 0.05.
Results
Demographic Characteristics
We first identified 8,675 patients with COVID‐19 (97.6% of all patients with COVID‐19 reported to the Department of Health, Hong Kong) from January 23, 2020, to January 1, 2021. We excluded 2,986 patients who had missing HBsAg results and 50 patients who had HCV infection; thus, 5,639 patients (353 current HBV infection, 359 past HBV infection, and 4,927 no known HBV exposure) were included in the final analysis (Fig. 1). At baseline, compared to patients with COVID‐19 without known HBV exposure, current HBV‐infected patients were older, had higher ALT and lower neutrophil and platelet counts, and were more likely to have cirrhosis. Past HBV‐infected patients with COVID‐19 were the oldest among the three groups of patients; they had higher creatinine, C‐reactive protein, lactate dehydrogenase, and neutrophil‐to‐lymphocyte ratio and were more likely to have DM and cardiovascular disease (Table 1).
FIG. 1.
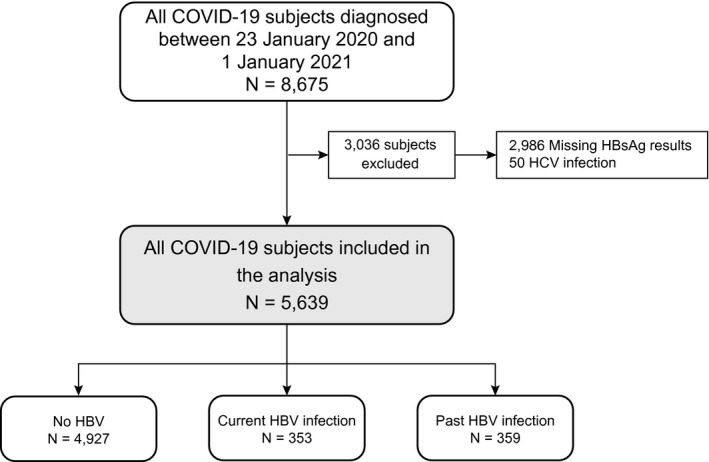
Selection of patients with SARS‐CoV‐2 infection/COVID‐19.
Table 1.
Baseline clinical characteristics of patients with SARS‐CoV‐2 infection/COVID‐19 who had no HBV infection, who had current HBV infection, and who had past HBV infection
| Clinical Characteristics | No HBV (n = 4,927) | Current HBV Infection (n = 353) | Past HBV Infection (n = 359) | P |
|---|---|---|---|---|
| Age (years) | 49.6 ± 18.4 | 56.2 ± 13.0 | 61.6 ± 14.2 | <0.001 |
| Male gender (n, %) | 2,387 (48.4) | 180 (51.0) | 176 (49.0) | 0.645 |
| Liver cirrhosis (n, %) | 43 (0.9) | 23 (6.5) | 13 (3.6) | <0.001 |
| Albumin (g/L) | 40.1 ± 5.3 | 39.5 ± 4.7 | 39.0 ± 5.3 | <0.001 |
| Missing (%) | 0.1 | 0 | 0 | |
| Total bilirubin (mg/dL) | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.5 ± 0.5 | 0.073 |
| Missing (%) | 0.1 | 0 | 0 | |
| ALT (U/L) | 25 (16‐39) | 28 (20‐39) | 23 (16‐34) | <0.001 |
| Missing (%) | 0.1 | 0 | 0 | |
| AST (U/L) | 30 (22‐48) | 32 (24‐45) | 30 (22‐52) | 0.819 |
| Missing (%) | 68.1 | 58.9 | 53.2 | |
| ALP (×ULN) | 0.6 (0.5‐0.7) | 0.6 (0.4‐0.7) | 0.6 (0.5‐0.7) | 0.013 |
| Missing (%) | 0.1 | 0 | 0 | |
| International normalized ratio | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.4 | 0.518 |
| Missing (%) | 34.0 | 30.3 | 20.9 | |
| Creatinine (μmol/L) | 70 (59‐84) | 71 (59‐86) | 75 (60‐92) | <0.001 |
| Missing (%) | 0.1 | 0 | 0 | |
| C‐reactive protein (mg/dL) | 1.9 ± 3.6 | 2.0 ± 3.7 | 2.8 ± 4.5 | 0.002 |
| Missing (%) | 1.1 | 0.6 | 0 | |
| Lactate dehydrogenase (U/L) | 220.1 ± 89.1 | 232.3 ± 88.0 | 251.9 ± 135.3 | <0.001 |
| Missing (%) | 1.2 | 1.1 | 0.6 | |
| Hemoglobin (g/dL) | 13.6 ± 1.7 | 13.6 ± 1.5 | 13.2 ± 1.9 | 0.002 |
| Missing (%) | 0.04 | 0 | 0 | |
| WCC (×109/L) | 5.7 ± 2.2 | 5.2 ± 2.3 | 5.5 ± 2.3 | <0.001 |
| WCC <3.5 × 109/L (n, %) | 520 (10.6) | 53 (15.0) | 57 (15.9) | 0.001 |
| Missing (%) | 0.04 | 0 | 0 | |
| Neutrophil (×109/L) | 3.7 ± 1.9 | 3.3 ± 1.9 | 3.7 ± 2.1 | <0.001 |
| Missing (%) | 0.4 | 0.6 | 0 | |
| Lymphocyte (×109/L) | 1.3 ± 0.7 | 1.3 ± 1.2 | 1.1 ± 0.6 | <0.001 |
| Lymphocyte <1 × 109/L (n, %) | 1,557 (31.7) | 127 (36.2) | 169 (47.1) | <0.001 |
| Missing (%) | 0.4 | 0.6 | 0 | |
| Neutrophil‐to‐lymphocyte ratio | 3.5 ± 3.2 | 3.3 ± 3.0 | 4.3 ± 5.4 | 0.006 |
| Missing (%) | 0.4 | 0.6 | 0 | |
| Platelet (×109/L) | 219.1 ± 74.2 | 188.5 ± 67.7 | 196.5 ± 67.6 | <0.001 |
| Platelet <150 × 109/L (n, %) | 761 (15.5) | 111 (31.4) | 85 (23.7) | <0.001 |
| Missing (%) | 0.04 | 0 | 0 | |
| Comorbidities (n, %) | ||||
| Circulatory system disease | 1,513 (30.7) | 118 (33.4) | 185 (51.5) | <0.001 |
| DM | 960 (19.5) | 78 (22.1) | 141 (39.3) | <0.001 |
| Malignant tumor | 175 (3.6) | 29 (8.2) | 40 (11.1) | <0.001 |
| Nervous system disease | 229 (4.6) | 15 (4.2) | 20 (5.6) | 0.671 |
| Respiratory disease | 205 (4.2) | 11 (3.1) | 21 (5.8) | 0.176 |
| Kidney disease | 102 (2.1) | 6 (1.7) | 30 (8.4) | <0.001 |
| HIV infection | 6 (0.1) | 2 (0.6) | 1 (0.3) | 0.066 |
| Medications during follow‐up (n, %) | ||||
| Oseltamivir | 70 (1.4) | 4 (1.1) | 12 (3.3) | 0.013 |
| Ribavirin | 1,454 (29.5) | 98 (27.8) | 151 (42.1) | <0.001 |
| Lopinavir–ritonavir | 1,542 (31.3) | 103 (29.2) | 112 (31.2) | 0.708 |
| Interferon‐beta | 2,318 (47.0) | 169 (47.9) | 227 (63.2) | <0.001 |
| Remdesivir | 395 (8.0) | 35 (9.9) | 46 (12.8) | 0.004 |
| Antibiotic treatment | 2,139 (43.4) | 167 (47.3) | 196 (54.6) | <0.001 |
| Antifungal treatment | 34 (0.7) | 1 (0.3) | 9 (2.5) | 0.003 |
| Corticosteroid | 1,044 (21.2) | 86 (24.4) | 140 (39.0) | <0.001 |
| Dexamethasone | 966 (19.6) | 79 (22.4) | 128 (35.7) | <0.001 |
| Hydrocortisone | 119 (2.4) | 7 (2.0) | 26 (7.2) | <0.001 |
| Prednisolone | 61 (1.2) | 2 (0.6) | 13 (3.6) | 0.001 |
| Methylprednisolone | 6 (0.1) | 2 (0.6) | 0 (0) | 0.118 |
| Peak daily dose (prednisolone equivalent, mg) | 45 (45‐45) | 45 (45‐53) | 45 (45‐58) | 0.364 |
| Duration (days) | 10 (7‐13) | 10 (6‐12) | 11 (6‐16) | 0.290 |
| Immunoglobulin therapy (i.v.) | 6 (0.1) | 2 (0.6) | 0 (0) | 0.118 |
| Oral HBV antiviral agents* | <0.001 | |||
| Entecavir | 70 (1.4) | 114 (32.3) | 40 (11.1) | |
| Tenofovir disoproxil fumarate/tenofovir alafenamide | 0 (0) | 6 (1.7) | 0 (0) | |
| Lamivudine ± adefovir | 1 (0.02) | 2 (0.6) | 0 (0) | |
| Clinical outcomes in 60 days (n, %) | ||||
| Mortality | 109 (2.2) | 8 (2.3) | 21 (5.8) | <0.001 |
| Follow‐up duration (days) | 13 (9‐20) | 14 (9‐20) | 16 (10‐25) | <0.001 |
All concomitant medications were represented as binary parameters. Percentages were computed based on nonmissing values. Categorical variables are presented as number (percentage). Median age, ALT, and follow‐up duration are expressed as median (IQR), whereas other continuous variables are expressed as mean ± SD. Qualitative and quantitative differences between subgroups were analyzed by chi‐squared or Fisher’s exact test for categorical parameters and Student t test or Mann‐Whitney U test for continuous parameters, as appropriate.
The 71 patients who used HBV antiviral agents in the non‐HBV group had negative HBsAg and unavailable anti‐HBc and anti‐HBs status.
Abbreviation: WCC, white cell count.
Current HBV infection
Among 166 patients with COVID‐19 and current HBV infection who had available HBeAg status, 91.6% were HBeAg‐negative. Among 225 current HBV‐infected patients who had available HBV DNA measurement, 78.2% had detectable HBV DNA with a median (IQR) of 68 (10‐1,360) IU/mL; 37 patients had HBV DNA > 2,000 IU/mL. Among 353 HBV patients, 122 (34.6%) received HBV antiviral treatment, among whom 73 initiated antiviral treatment after COVID‐19 diagnosis. Among the 73 patients, 48 started antiviral treatment due to prophylaxis during corticosteroid therapy; 16 were started due to elevated ALT above ULN (8 with HBV DNA > 2,000 IU/mL); 9 were started for other reasons.
Past HBV infection
Among 359 patients with past HBV infection, 40 (11.1%) received antiviral treatment; 31 were due to prophylaxis during corticosteroid therapy, 2 were due to prophylaxis during chemotherapy, and 7 remained on treatment after HBsAg seroclearance. The 71 patients who used HBV antiviral agents in the non‐HBV group had negative HBsAg and unavailable anti‐HBc and anti‐HBs status.
Liver test abnormalities and liver injury
ALT abnormality occurred in 2,394 (48.6%), 187 (53.0%), and 194 (54.0%) patients with no, current, and past HBV infection, respectively (chi‐squared test, P = 0.001) (Table 2 and Fig. 2A; Supporting Fig. S1A). Abnormal total bilirubin occurred in 1,123 (22.8%), 77 (21.8%), and 110 (30.6%) patients with no, current, and past HBV infection, respectively (chi‐squared test, P = 0.001) (Fig. 2B; Supplementary Fig. S1B). Abnormal ALP occurred in 549 (11.1%), 34 (9.6%), and 61 (17.0%) patients with no, current, and past HBV infection, respectively (chi‐squared test, P = 0.003) (Fig. 2C). Acute liver injury occurred in 58 (1.2%), 8 (2.3%), and 11 (3.1%) patients with no, current, and past HBV infection, respectively (Fisher’s exact test, P = 0.005). Liver‐related morbidity during COVID‐19 was uncommon; no patients had liver‐related death (Supporting Table S5). The 233 patients with COVID‐19 who had received HBV antiviral treatment were older, had more comorbidities, and had poorer renal and liver function than patients who did not receive HBV antiviral treatment. More patients who had received HBV antiviral treatment had acute liver injury and mortality (Supporting Table S6).
Table 2.
Abnormal liver biochemistries during follow‐up among all patients with SARS‐CoV‐2 infection/COVID‐19 who had no HBV infection, who had current HBV infection, and who had past HBV infection
| No HBV (n = 4,927) | Current HBV Infection (n = 353) | Past HBV Infection (n = 359) | P | |
|---|---|---|---|---|
| Acute liver injury | 58 (1.2) | 8 (2.3) | 11 (3.1) | 0.005 |
| Peak ALT | ||||
| < ULN | 2,533 (51.4) | 166 (47.0) | 165 (46.0) | 0.001 |
| ≥1 × to <2 × ULN | 1,245 (25.3) | 105 (29.7) | 88 (24.5) | |
| ≥2 × to <5 × ULN | 909 (18.4) | 69 (19.5) | 79 (22.0) | |
| ≥5 × to <10 × ULN | 194 (3.9) | 7 (2.0) | 16 (4.5) | |
| ≥10 × ULN | 46 (0.9) | 6 (1.7) | 11 (3.1) | |
| Peak ALP | ||||
| < ULN | 4,378 (88.9) | 319 (90.4) | 298 (83.0) | 0.003 |
| ≥1 × to <2 × ULN | 485 (9.8) | 30 (8.5) | 47 (13.1) | |
| ≥2 × to <5 × ULN | 58 (1.2) | 4 (1.1) | 14 (3.9) | |
| ≥5 × ULN | 6 (0.1) | 0 (0) | 0 (0) | |
| Peak total bilirubin | ||||
| < ULN | 3,804 (77.2) | 276 (78.2) | 249 (69.4) | 0.001 |
| ≥1 × to <2 × ULN | 953 (19.3) | 60 (17.0) | 85 (23.7) | |
| ≥2 × to <5 × ULN | 151 (3.1) | 15 (4.2) | 19 (5.3) | |
| ≥5 × to <10 × ULN | 12 (0.2) | 2 (0.6) | 5 (1.4) | |
| ≥10 × ULN | 7 (0.1) | 0 (0) | 1 (0.3) | |
| Peak AST* | n = 1,574 | n = 145 | n = 168 | |
| <ULN | 929 (59.0) | 84 (57.9) | 87 (51.8) | 0.134 |
| ≥1 × to <2 × ULN | 379 (24.1) | 45 (31.0) | 41 (24.4) | |
| ≥2 × to <5 × ULN | 217 (13.8) | 13 (9.0) | 32 (19.0) | |
| ≥5 × to <10 × ULN | 33 (2.1) | 2 (1.4) | 6 (3.6) | |
| ≥10 × ULN | 16 (1.0) | 1 (0.7) | 2 (1.2) | |
| Peak GGT* | n = 938 | n = 88 | n = 118 | |
| <ULN | 349 (37.2) | 44 (50.0) | 48 (40.7) | 0.055 |
| ≥1 × to <2 × ULN | 239 (25.5) | 28 (31.8) | 32 (27.1) | |
| ≥2 × to <5 × ULN | 222 (23.7) | 11 (12.5) | 27 (22.9) | |
| ≥5 × to <10 × ULN | 87 (9.3) | 4 (4.5) | 6 (5.1) | |
| ≥10 × ULN | 41 (4.4) | 1 (1.1) | 5 (4.2) |
For peak liver function tests during follow‐up, patients were followed from the date of COVID‐19 diagnosis to the date of discharge, the last follow‐up date (January 20, 2021), or date of death, whichever came first.
Acute liver injury was defined as ALT and/or AST ≥ 2 × ULN, with total bilirubin ≥2 × ULN and/or international normalized ratio ≥1.7.
Percentages were based on nonmissing data.
Abbreviation: GGT, gamma‐glutamyl transferase.
FIG. 2.
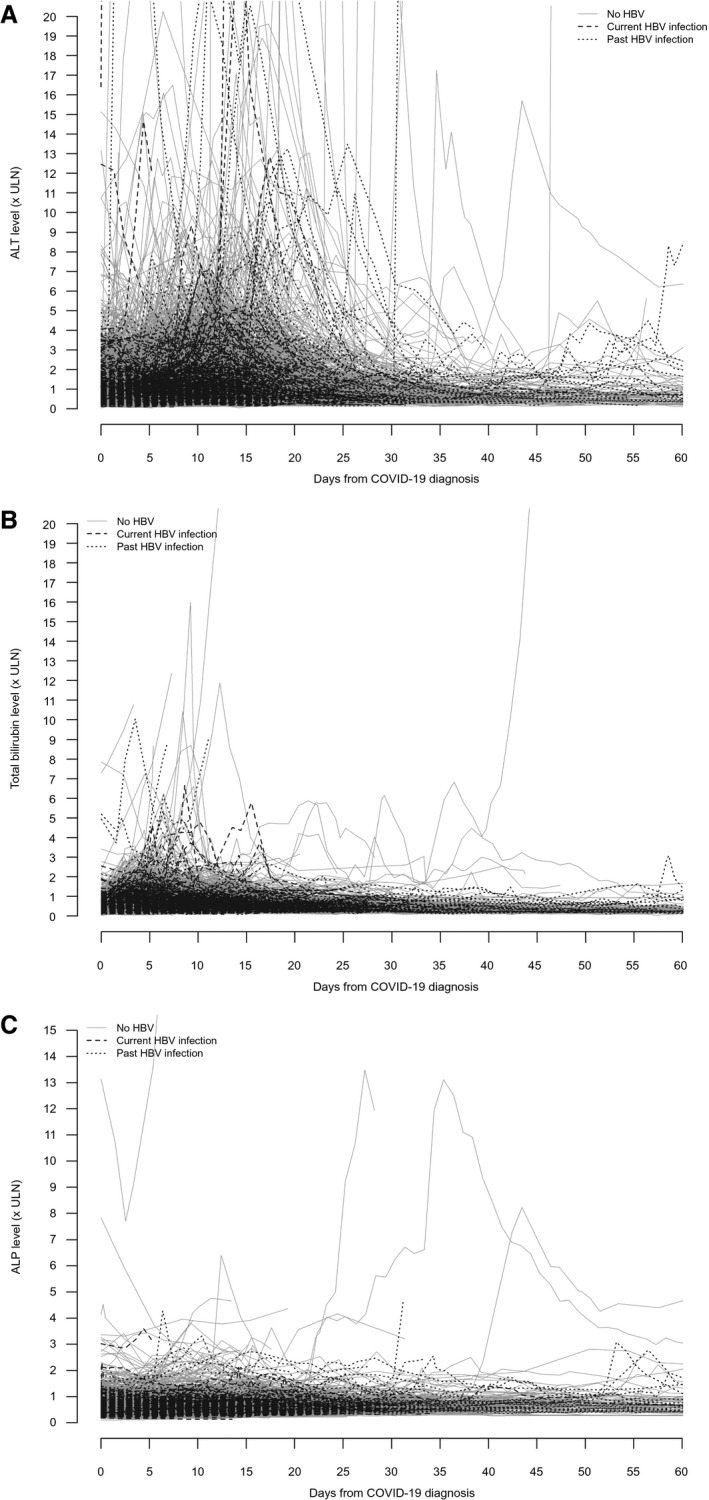
Serial (A) ALT, (B) total bilirubin, and (C) ALP of patients with SARS‐CoV‐2 infection/COVID‐19 who had no HBV infection, who had current HBV infection, or who had past HBV infection.
CHB, liver injury, and clinical outcomes
Mortality was observed in 109 (2.2%), 8 (2.3%), and 21 (5.8%) patients with no, current, and past HBV infection, respectively (Table 1). On univariate analysis, past HBV infection (HR, 1.97; 95% CI, 1.23‐3.15; P = 0.005), but not current HBV infection (HR, 1.10; 95% CI, 0.53‐2.25; P = 0.802), was associated with mortality. However, neither current (aHR, 1.09; 95% CI, 0.52‐2.27; P = 0.829) nor past (aHR, 1.05; 95% CI, 0.65‐1.69; P = 0.836) HBV infections was associated with mortality on multivariable analysis after adjusting for age, presence of acute liver injury, liver cirrhosis, DM, malignant tumor, nervous system disease, and kidney disease (Table 3). Acute liver injury and presence of liver cirrhosis were independently associated with higher risk of mortality (Table 3; Supporting Table S4). Similar results were observed after adjusting for relevant laboratory parameters (Supporting Table S4).
Table 3.
Univariate and multivariable analyses with Cox proportional hazards regression on factors associated with mortality in patients with SARS‐CoV‐2 infection/COVID‐19.
| Parameters | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P | aHR (95% CI) | P | |
| HBV exposure | ||||
| No HBV | Referent | |||
| Current HBV infection | 1.10 (0.53‐2.25) | 0.802 | 1.09 (0.52‐2.27) | 0.829 |
| Past HBV infection | 1.97 (1.23‐3.15) | 0.005 | 1.05 (0.65‐1.69) | 0.836 |
| Acute liver injury | 6.87 (4.38‐10.78) | <0.001 | 3.11 (1.97‐4.92) | <0.001 |
| Liver cirrhosis | 4.35 (2.29‐8.29) | <0.001 | 2.36 (1.20‐4.63) | 0.013 |
| Age (years) | 1.11 (1.09‐1.12) | <0.001 | 1.09 (1.07‐1.11) | <0.001 |
| Male sex | 1.17 (0.83‐1.64) | 0.362 | ||
| Circulatory system disease | 11.17 (6.62‐18.87) | <0.001 | ||
| DM | 6.00 (4.13‐8.72) | <0.001 | 1.94 (1.31‐2.88) | 0.001 |
| Malignant tumor | 5.40 (3.60‐8.11) | <0.001 | 1.80 (1.17‐2.77) | 0.007 |
| Nervous system disease | 4.98 (3.42‐7.25) | <0.001 | 2.13 (1.45‐3.13) | <0.001 |
| Respiratory disease | 3.93 (2.62‐5.89) | <0.001 | ||
| Kidney disease | 7.42 (4.96‐11.10) | <0.001 | 2.27 (1.48‐3.48) | <0.001 |
Patients were followed from the date of COVID‐19 diagnosis to the date of discharge, the last follow‐up date (20 January 2021), or date of death, whichever came first.
Acute liver injury was defined as ALT and/or AST ≥ 2 × ULN, with total bilirubin ≥2 × ULN and/or international normalized ratio ≥1.7.
IPTW by PS improved the similarity of distributions of the 21 clinical characteristics between patients with current, past, and no HBV infections and reduced all ASMDs to <0.2; Table 4 shows the result in one of the 20 imputed data sets; consistent patterns were obtained across other imputed data sets. After IPTW, neither current (weighted HR, 1.32; 95% CI, 0.63‐2.78; P = 0.463) nor past (weighted HR, 0.97; 95% CI, 0.54‐1.77; P = 0.929) HBV infections was associated with mortality, with reference to patients with no HBV infection (Fig. 3).
Table 4.
Baseline clinical characteristics and balancing diagnostics in PS weighting analysis between patients with SARS‐CoV‐2 infection/COVID‐19 who had no HBV infection, who had current HBV infection, and who had past HBV infection for a single multiple imputation data set
| Clinical Characteristics | Before PS Weighting | After PS Weighting | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No HBV (n = 4,927) | Current HBV Infection (n = 353) | Past HBV Infection (n = 359) | ASMD* | ASMD † | No HBV (n = 286) | Current HBV Infection (n = 353) | Past HBV Infection (n = 165) | ASMD* | ASMD † | |
| Age (years) | 49.6 ± 18.4 | 56.2 ± 13.0 | 61.6 ± 14.2 | 0.51 | 0.42 | 55.5 ± 13.7 | 56.2 ± 13.0 | 57.7 ± 12.5 | 0.05 | 0.12 |
| Male sex (n, %) | 2,387 (48.4) | 178 (51.0) | 176 (49.0) | 0.05 | 0.04 | 141 (49.3) | 180 (51.0) | 78 (47.3) | 0.03 | 0.07 |
| Liver cirrhosis (n, %) | 43 (0.9) | 23 (6.5) | 13 (3.6) | 0.23 | 0.12 | 7 (2.4) | 23 (6.5) | 6 (3.6) | 0.16 | 0.13 |
| Comorbidities (n, %) | ||||||||||
| Cardiovascular diseases | 1,513 (30.7) | 118 (33.4) | 185 (51.5) | 0.06 | 0.38 | 99 (34.6) | 118 (33.4) | 63 (38.2) | 0.03 | 0.10 |
| DM | 960 (19.5) | 78 (22.1) | 141 (39.3) | 0.06 | 0.41 | 65 (22.6) | 78 (22.1) | 43 (26.1) | 0.01 | 0.10 |
| Malignant tumor | 175 (3.6) | 29 (8.2) | 40 (11.1) | 0.17 | 0.11 | 13 (4.5) | 29 (8.2) | 14 (8.5) | 0.13 | 0.02 |
| Nervous system diseases | 229 (4.6) | 15 (4.2) | 20 (5.6) | 0.02 | 0.07 | 13 (4.5) | 15 (4.2) | 7 (4.2) | 0.02 | 0.01 |
| Respiratory disease | 205 (4.2) | 11 (3.1) | 21 (5.8) | 0.06 | 0.16 | 11 (3.8) | 11 (3.1) | 6 (3.6) | 0.03 | 0.04 |
| Kidney disease | 102 (2.1) | 6 (1.7) | 30 (8.4) | 0.03 | 0.51 | 5 (1.7) | 6 (1.7) | 6 (3.6) | 0.004 | 0.13 |
| Laboratory results | ||||||||||
| Creatinine (µmol/L) | 70 (59‐84) | 71 (59‐86) | 75 (60‐92) | 0.09 | 1.13 | 71 (59‐86) | 71 (59‐86) | 71 (60‐74) | 0.06 | 0.19 |
| Albumin (g/L) | 40.1 ± 5.3 | 39.5 ± 4.7 | 39.0 ± 5.3 | 0.13 | 0.11 | 39.6 ± 4.7 | 39.5 ± 4.7 | 39.5 ± 4.5 | 0.03 | 0.005 |
| ALT (U/L) | 25 (16‐39) | 28 (20‐39) | 23 (16‐34) | 0.08 | 0.15 | 27 (19‐40) | 28 (20‐39) | 25 (18‐38) | 0.05 | 0.11 |
| ALP (×ULN) | 0.6 (0.5‐0.7) | 0.6 (0.4‐0.7) | 0.6 (0.5‐0.7) | 0.16 | 0.12 | 0.6 (0.4‐0.7) | 0.6 (0.4‐0.7) | 0.6 (0.4‐0.7) | 0.02 | 0.01 |
| Total bilirubin (mg/dL) | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.5 ± 0.5 | 0.09 | 0.07 | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.5 ± 0.4 | 0.05 | <0.001 |
| LDH (U/L) | 220.2 ± 89.0 | 233.7 ± 92.8 | 252.2 ± 135.1 | 0.15 | 0.20 | 228.4 ± 87.7 | 233.7 ± 92.8 | 234.2 ± 98.5 | 0.06 | 0.006 |
| CRP (mg/dL) | 1.9 ± 3.7 | 2.0 ± 3.6 | 2.8 ± 4.5 | 0.02 | 0.22 | 1.9 ± 3.5 | 2.0 ± 3.6 | 2.2 ± 3.5 | 0.01 | 0.05 |
| Hemoglobin (g/dL) | 13.6 ± 1.7 | 13.6 ± 1.5 | 13.2 ± 1.9 | 0.05 | 0.28 | 13.6 ± 1.6 | 13.6 ± 1.5 | 13.4 ± 1.5 | 0.01 | 0.14 |
| White cell count (×109/L) | 5.7 ± 2.2 | 5.2 ± 2.3 | 5.5 ± 2.3 | 0.21 | 0.10 | 5.2 ± 1.9 | 5.2 ± 2.3 | 5.2 ± 1.9 | 0.003 | 0.03 |
| Lymphocyte (×109/L) | 1.3 ± 0.7 | 1.3 ± 1.2 | 1.1 ± 0.6 | 0.03 | 0.13 | 1.3 ± 0.6 | 1.3 ± 1.2 | 1.2 ± 0.7 | 0.04 | 0.06 |
| Neutrophil (×109/L) | 3.7 ± 1.9 | 3.3 ± 1.9 | 3.7 ± 2.1 | 0.22 | 0.22 | 3.4 ± 1.7 | 3.3 ± 1.9 | 3.4 ± 1.7 | 0.02 | 0.03 |
| Platelet (×109/L) | 219.1 ± 74.3 | 188.5 ± 67.7 | 196.5 ± 67.6 | 0.45 | 0.12 | 194.6 ± 66.4 | 188.5 ± 67.7 | 196.1 ± 63.0 | 0.09 | 0.11 |
| Follow‐up duration (days) | 13 (9‐20) | 14 (9‐20) | 16 (10‐25) | — | — | 14 (10‐19) | 14 (9‐20) | 15 (11‐23) | — | — |
An ASMD < 0.2 indicated good balance between the two groups. Parameters with ASMD ≥ 0.2 would be adjusted in the doubly robust model.
Comparing patients with and without HBV infection.
Comparing patients with current and past HBV infection.
Abbreviations: CRP, C‐reactive protein; LDH, lactate dehydrogenase.
FIG. 3.
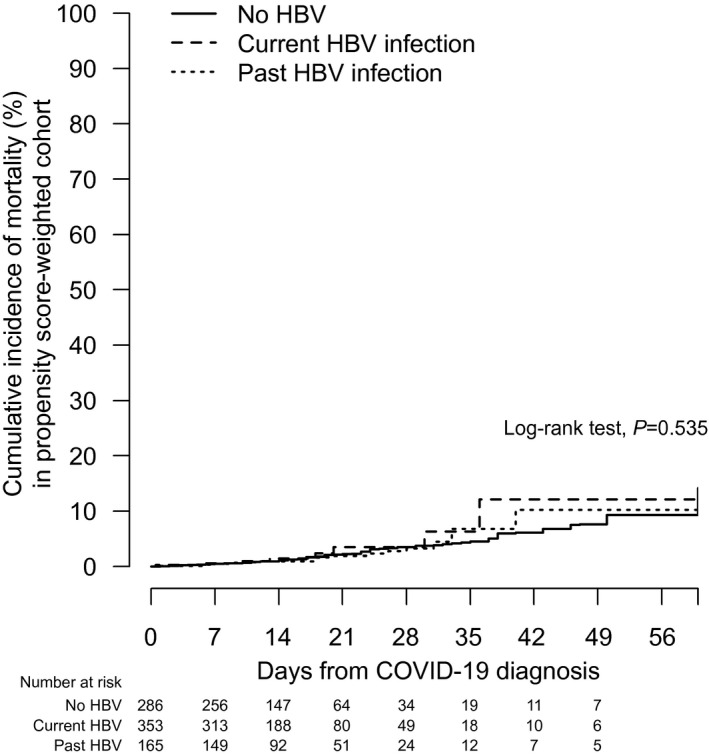
Cumulative incidence of mortality after PS weighting in patients with SARS‐CoV‐2 infection/COVID‐19 who had no HBV infection, who had current HBV infection, or who had past HBV infection in a single multiple imputation data set.
Safety of comedications in patients with COVID‐19 with current, past, and no HBV infections
Advanced age, male gender, presence of DM, and use of corticosteroids, antifungals, lopinavir–ritonavir, and ribavirin were independently associated with acute liver injury in patients with COVID‐19 (Table 5). Presence of current (aOR, 1.93; 95% CI, 0.88‐4.24; P = 0.102) or past (aOR, 1.25; 95% CI, 0.62‐2.55; P = 0.533) HBV infection was not associated with acute liver injury.
Table 5.
Univariate and multivariable analyses by logistic regression on factors associated with acute liver injury in patients with SARS‐CoV‐2 infection/COVID‐19
| Parameters | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P | aOR (95% CI) | P | |
| HBV exposure | ||||
| No HBV | Referent | |||
| Current HBV infection | 1.95 (0.92‐4.11) | 0.081 | 1.93 (0.88‐4.24) | 0.102 |
| Past HBV infection | 2.65 (1.38‐5.10) | 0.003 | 1.25 (0.62‐2.55) | 0.533 |
| Age | 1.05 (1.04‐1.07) | <0.001 | 1.03 (1.01‐1.05) | 0.003 |
| Male gender | 3.27 (1.94‐5.51) | <0.001 | 2.40 (1.40‐4.12) | 0.002 |
| Liver cirrhosis | 1.90 (0.46‐7.88) | 0.377 | ||
| DM | 7.27 (4.53‐11.67) | <0.001 | 2.27 (1.32‐3.91) | 0.003 |
| Use of corticosteroid | 10.22 (6.12‐17.07) | <0.001 | 3.29 (1.83‐5.91) | <0.001 |
| Use of remdesivir | 1.83 (0.96‐3.48) | 0.067 | ||
| Use of interferon‐beta | 4.94 (2.76‐8.84) | <0.001 | ||
| Use of ribavirin | 2.82 (1.79‐4.43) | <0.001 | 2.55 (1.57‐4.14) | <0.001 |
| Use of lopinavir/ritonavir | 4.18 (2.61‐6.70) | <0.001 | 3.20 (1.94‐5.27) | <0.001 |
| Use of antibiotics | 6.95 (3.74‐12.89) | <0.001 | ||
| Use of antifungals | 31.90 (15.73‐64.70) | <0.001 | 5.63 (2.55‐12.45) | <0.001 |
Acute liver injury was defined as ALT and/or AST ≥ 2 × ULN, with total bilirubin ≥2 × ULN and/or international normalized ratio ≥1.7.
P = 0.572 for Hosmer‐Lemeshow goodness‐of‐fit test, which did not indicate a poor fit.
Among 353 patients with COVID‐19 with current HBV infection, the median (IQR) ALT at baseline was 27 (20‐39) and 29 (20‐42) U/L in patients who received and did not receive nucleos(t)ide analogue (NA) treatment, respectively (Mann‐Whitney test, P = 0.193). The median (IQR) of peak ALT was 38 (25‐63) and 54 (31‐116) in untreated and NA‐treated patients, respectively (Quade’s ANCOVA, P < 0.001). At discharge, the median (IQR) of ALT was 33 (21‐49) and 32 (22‐61) who received and did not receive NA treatment, respectively (Quade’s ANCOVA, P = 0.524). Of 353 patients, 5/122 (4.1%) and 3/231 (1.3%) who received and did not receive NA treatment developed acute liver injury, respectively (Fisher’s exact test, P = 0.131).
Moreover, 86/353 (24.4%) patients with COVID‐19 with current HBV infection received corticosteroid (79 dexamethasone, 7 hydrocortisone, 2 prednisolone, 2 methylprednisolone [patients might have used more than one type of steroid]); 60/86 (69.8%) were also receiving NA treatment (56 entecavir, 3 tenofovir disoproxil fumarate, 1 lamivudine, and adefovir dipivoxil); 48 and 12 used that before and after COVID‐19 diagnosis, respectively. The median (IQR) ALT before corticosteroid therapy was 25 (18‐36) and 33 (24‐52) U/L in patients who received and did not receive NA treatment, respectively (Mann‐Whitney test, P = 0.034). The median (IQR) of peak ALT during or after corticosteroid therapy was 58 (31‐95) and 64 (42‐138) in patients who received and did not receive NA treatment, respectively (Quade’s ANCOVA, P = 0.851). Two of 60 (3.3%) and 2/26 (7.7%) of the patients who used and did not use NA developed acute liver injury, respectively (Fisher’s exact test, P = 0.581). Among 26 patients who had severe COVID‐19, i.e., with admission to an intensive care unit or mortality, and received corticosteroid therapy, 1/17 (5.9%) and 2/9 (22.2%) who used and did not use NA developed acute liver injury, respectively (Fisher’s exact test, P = 0.268). Among 10 patients who had HBV DNA measurement before and after corticosteroid therapy, all were on NA treatment; no one had evidence of HBV reactivation (i.e., HBV DNA increased for more than one log10 IU/mL from baseline).
For 359 patients with past HBV infection, 140 (39.0%) received corticosteroid (128 dexamethasone, 26 hydrocortisone, 13 prednisolone [patients might have used more than one type of steroid]); 32/140 (22.9%) were also receiving NA treatment. Three of 32 (9.4%) and 7/108 (6.5%) of the patients who used and did not use NA developed acute liver injury, respectively (Fisher’s exact test, P = 0.696).
Discussion
We report the incidence and pattern of liver injury in patients with COVID‐19 with current or past HBV infection or without HBV infection. This is one of the largest cohorts of patients with COVID‐19 with current and past HBV infection who had serial measurements on liver biochemistries. The liver biochemistries of patients with COVID‐19 changed dynamically during the clinical course, yet there was no obvious difference between patients with COVID‐19 with current, past, and no HBV infection. While acute liver injury occurred in 58 (1.2%), 8 (2.3%), and 11 (3.1%) patients with no, current, and past HBV infection, respectively, current and past HBV infections were not associated with acute liver injury after adjusting for patients’ demographics and use of medications for COVID‐19. On the other hand, acute liver injury was shown to be independently associated with mortality in patients with COVID‐19 independent of HBV infection status, consistent with previous studies.( 3 , 4 , 5 )
While liver injury is a well‐known phenomenon in patients with COVID‐19, so far the exact impact of COVID‐19 on patients with current and past HBV infection has not been well elucidated. In patients with CHB and COVID‐19, ALT/AST elevation may be secondary to HBV reactivation( 7 ) or reactive hepatitis in the presence of systematic inflammatory response.( 2 ) While it would be difficult to tease out the exact causes of hepatitis flare in patients with COVID‐19 with HBV infection, it would be reasonable to initiate HBV antiviral treatment for patients with current HBV infection whenever they fulfill the treatment criteria recommended by international guidelines, namely HBV DNA > 2,000 IU/mL with ALT > ULN( 33 ) or 2 × ULN,( 19 , 34 ) and compensated or decompensated cirrhosis with detectable HBV DNA.( 19 , 33 , 34 ) In our cohort, 73 treatment‐naive patients with CHB started HBV antiviral treatment during SARS‐CoV‐2 infection; 48 patients started due to prophylaxis during corticosteroid therapy, and 16 started due to elevated ALT above ULN (8 with HBV DNA > 2,000 IU/mL).
The safety of COVID‐19 therapies in patients with HBV infection has been a concern as systemic high‐dose corticosteroids, which are immunomodulators and may lead to HBV reactivation, are now the standards of care for critically ill patients with COVID‐19.( 11 , 35 ) HBV reactivation potentially results in life‐threatening hepatitis flare and acute liver failure in HBV‐infected patients.( 36 ) Hence, it is advocated for patients with COVID‐19 to screen for HBsAg; antiviral prophylaxis with NAs is recommended in all HBsAg‐positive patients with severe COVID‐19 during corticosteroid therapy.( 12 ) In our cohort, 70% of patients with current HBV infection received antiviral prophylaxis during corticosteroid therapy; among 26 patients with severe COVID‐19 and corticosteroid therapy, 6% and 22% who used and did not use NA developed acute liver injury, respectively. While the absolute number of patients was small, antiviral prophylaxis may be important for HBsAg‐positive patients with COVID‐19 during corticosteroid therapy. While patients with COVID‐19 and current HBV infection who received NA treatment had a higher peak ALT than those who did not receive NA, the ALT level at discharge was comparable between patients who received and did not receive NA.
For patient with past HBV infection, 11% received NA during COVID‐19, mainly due to prophylaxis during corticosteroid therapy. Past HBV‐infected patients had more acute liver injury as shown by univariate analysis, yet the association was no longer significant after adjusting for their age, gender, presence of DM, and use of corticosteroid and other medications for COVID‐19. After all, acute liver injury and liver‐related morbidity and mortality are uncommon and may not be significantly contributed to by current or past HBV infection in patients with COVID‐19. Provided that patients with current HBV infection who fulfill HBV treatment criteria or are under corticosteroid therapy receive NA treatment, NA prophylaxis in all patients with current or even past HBV infection once diagnosed with COVID‐19 may not be necessary. Yet, vigilant monitoring of ALT and HBV DNA remains important during SARS‐CoV‐2 infection, to guide the use of NA for these patients. On the other hand, vigilant monitoring of patients with acute liver injury or liver cirrhosis may also be important as they were shown to have higher risk of mortality.
As in other patients with COVID‐19, those with current or past HBV infection may have other reasons leading to liver injury, namely ischemic hepatitis from hypoxemia and hypotension, sepsis, and DILI.( 37 ) Many of the drugs being used in severe COVID‐19 cases are associated with hepatotoxicity, including COVID‐19 therapies, antibiotics, and antifungal agents. This association could be due to more severe disease in patients with COVID‐19 who received such combination COVID‐19 therapy. HBV is known to increase the risk of DILI according to the “danger hypothesis,” where the role of costimulatory triggers is an essential step in the pathogenesis of DILI as the cytokines released by stressed or dead cells provide additional stimulation to the antigen‐presenting cell, which leads to a further recruitment of helper and cytotoxic T cells, culminating in antibody‐dependent cell‐mediated cytotoxicity.( 38 ) Yet in our current study, the risk of liver injury is not increased in patients with current or past HBV infection. Hence, the contribution by HBV to DILI is unlikely to be substantial. Also, current and past HBV infections are not associated with the development of adverse clinical outcomes, whereas liver injury is associated with the development of adverse clinical outcomes. In fact, the majority of patients with current HBV infection in our study had low‐level HBV viremia.
A strength of our study is the use of a territory‐wide cohort that covered 97.6% of COVID‐19 cases in Hong Kong, where HBV remains endemic. A majority of these patients had HBsAg checked, whereas around half of the patients who did not have their HBsAg checked were born in the era of HBV universal vaccination. We analyzed data from patients with current, past, and no HBV infections so that the exact role of current and past HBV infection in COVID‐19 could be demonstrated. Data from real‐life cohorts represent a wider spectrum of patients such that the findings from real‐life cohorts are thus more readily applicable to routine clinical practice. Nonetheless, our study has a few limitations. First, we missed 214 out of 8,889 (2.4%) patients with COVID‐19 as their SARS‐CoV‐2 results were not retrievable. Nonetheless, we believe missing 2.4% of the patients with COVID‐19 would not have a major impact on the findings as the proportion of deaths in our cohort (162/8,675, 1.9%) was consistent with what was reported officially (165/8,889, 1.9%). Second, there was potential misclassification of patients in the groups of no and past HBV infection. In our study, 71 patients who had negative HBsAg and missing anti‐HBc status received HBV antiviral treatment. Some of these patients may have a self‐reported HBV infection and were thus given antiviral treatment until a negative HBsAg test was found subsequently. Also, some of these patients may have undocumented past HBV infection and thus could be misclassified as no HBV infection. Also, there may be patients who were born before the launch of the universal neonatal vaccination program in Hong Kong in 1988 but had positive anti‐HBs due to subsequent HBV vaccination; they can thus be misclassified as patients with past HBV infection. The same definition to classify patients with no or past HBV infection had been adopted previously.( 13 ) Third, we defined cirrhosis by ICD‐9‐CM diagnosis codes for cirrhosis and its related complications and/or platelet counts <100 × 109/L in a measurement at least 30 days before COVID‐19 diagnosis. In real‐life clinical practice, physicians may use different methods to diagnose liver cirrhosis, which may affect the diagnosis coding in the computer system. Yet, it would be unrealistic to perform liver biopsies in patients with COVID‐19 and abnormal liver tests. Also, serum fibrosis scores including Fibrosis‐4 index and AST‐to‐platelet ratio index as well as transient elastography are unreliable in patients with acute liver injury.( 39 , 40 ) We acknowledged that some patients may have undiagnosed liver cirrhosis,( 41 ) though this can be partly reflected by patients’ platelet counts. We have also examined more definable ICD‐9‐CM codes for cirrhotic complications, which do not rely on a more accurate diagnosis of cirrhosis to identify the presence of cirrhosis. Fourth, missing laboratory measurements might lead to biases as in other retrospective studies, though these biases can partially be compensated for by our respectable cohort size. Some less common laboratory parameters might not be checked for every single patient due to minor variations of clinical practice in different hospitals. Yet, missing data were rare for common laboratory parameters including ALT, total bilirubin, and ALP as those are regularly checked in our routine clinical practice. Missing test on anti‐HCV was found in 3,997/8,675 (46.1%) of patients with COVID‐19. As the prevalence of HCV in Hong Kong is low (0.3% in the general population and 3.6% among patients with chronic HBV infection),( 42 , 43 ) the impact on our findings would be relatively small. In this study, we excluded 50 patients who had active or past HCV infection. In Hong Kong, 85% of the anti‐HCV‐positive patients have detectable HCV RNA.( 44 ) We acknowledged that patients with active or past HCV infection may have different outcomes with COVID‐19 infection, though previous studies suggest that patients with COVID‐19 and HCV infection do not have increased intensive care unit admission or mortality.( 45 ) Also, ascertainment bias may affect the reliability of the study due to inaccurate entry of certain diagnosis codes for comorbidities. We minimized this bias by including diagnosis, laboratory, as well as medication data for DM and hypertension.
In conclusion, patients with COVID‐19 with current, past, and no HBV infections have similar risk of liver injury. Current or past HBV infections are not associated with higher risk of mortality in patients with COVID‐19. There is no increased risk of DILI or virological flare of HBV. We observed generally good safety of most COVID‐19 therapies in patients with current and past HBV infection. Nonetheless, as liver injury per se is prognostic, we recommend vigilant monitoring of liver biochemistries and HBV DNA and cautious use of appropriate medications with the least hepatotoxicity to minimize such liver injury. Appropriate use of antiviral treatment for HBV during corticosteroid therapy for COVID‐19 would minimize the risk of HBV reactivation and acute liver injury.
Author Contributions
T.Y., V.W., G.L., H.C., D.H., and G.W. were responsible for the study concept and design. T.Y., Y.‐K.T., V.H., and G.W. were responsible for the acquisition and analysis of data, had full access to all of the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors were responsible for the interpretation of data and the drafting and critical revision of the manuscript for important intellectual content.
Supporting information
Supplementary Material
Supported by the Commissioned Health and Medical Research Fund of the Food and Health Bureau of the HKSAR government (ref. no. CID‐CUHK‐D).
Potential conflict of interest: Dr. Vincent Wong advises, is on the speakers’ bureau for, and received grants from Gilead. He advises and is on the speakers’ bureau for Echosens. He advises 3V‐BIO, AbbVie, Allergan, Boehringer Ingelheim, Intercept, Janssen, Novartis, Novo Nordisk, Perspectum Diagnostics, Pfizer, TARGET‐NASH, and Terns. He is on the speakers’ bureau for Bristol‐Myers Squibb and Merck. Dr. Lui consults for, advises, and received grants from Gilead. She advises and received grants from ViiV and MSD. Dr. Chan advises and is on the speakers’ bureau for Gilead and Roche. He advises AbbVie, Aptorum, Arbutus, Hepion, Intellia, Janssen, GlaxoSmithKline, GRAIL, Medimmune, Merck, Vaccitech, VenatoRx, and Vir Biotechnology. He is on the speakers’ bureau for Mylan. Dr. Grace Wong advises, is on the speakers’ bureau for, and received grants from Gilead. She advises and is on the speaker’s bureau for Janssen. She is on the speaker’s bureau for Abbott, Abbvie, Bristol‐Myers Squibb, Echosens, Furui, and Roche.
References
Author names in bold designate shared co‐first authorship.
- 1. World Health Organization . Weekly epidemiological update on COVID‐19—30 March 2021. https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐on‐covid‐19‐‐‐31‐march‐2021. Accessed April 4, 2021.
- 2. Wong G‐H, Wong V‐S, Thompson A, Jia J, Hou J, Lesmana CRA, et al. Management of patients with liver derangement during the COVID‐19 pandemic: an Asia‐Pacific position statement. Lancet Gastroenterol Hepatol 2020;5:776‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yip T‐F, Lui G‐Y, Wong V‐S, Chow V‐Y, Ho T‐Y, Li T‐M, et al. Liver injury is independently associated with adverse clinical outcomes in patients with COVID‐19. Gut 2021;70:733‐742. [DOI] [PubMed] [Google Scholar]
- 4. Weber S, Hellmuth JC, Scherer C, Muenchhoff M, Mayerle J, Gerbes AL. Liver function test abnormalities at hospital admission are associated with severe course of SARS‐CoV‐2 infection: a prospective cohort study. Gut 2021. Jan 29. 10.1136/gutjnl-2020-323800. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ding Z‐Y, Li G‐X, Chen L, Shu C, Song J, Wang W, et al. Association of liver abnormalities with in‐hospital mortality in patients with COVID‐19. J Hepatol 2021;74:1295‐1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Razavi‐Shearer D, Gamkrelidze I, Nguyen MH, Chen D‐S, Van Damme P, Abbas Z, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol 2018;3:383‐403. [DOI] [PubMed] [Google Scholar]
- 7. Zou X, Fang M, Li S, Wu L, Gao B, Gao H, et al. Characteristics of liver function in patients with SARS‐CoV‐2 and chronic HBV coinfection. Clin Gastroenterol Hepatol 2021;19:597‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He Q, Zhang G, Gu YE, Wang J, Tang Q, Jiang Z, et al. Clinical characteristics of COVID‐19 patients with pre‐existing hepatitis B virus infection: a multicenter report. Am J Gastroenterol 2021;116:420‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen L, Huang S, Yang J, Cheng X, Shang Z, Lu H, et al. Clinical characteristics in patients with SARS‐CoV‐2/HBV co‐infection. J Viral Hepat 2020;27:1504‐1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu R, Zhao LI, Cheng X, Han H, Li C, Li D, et al. Clinical characteristics of COVID‐19 patients with hepatitis B virus infection—a retrospective study. Liver Int 2021;41:720‐730. [DOI] [PubMed] [Google Scholar]
- 11. Group ; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid‐19—preliminary report. N Engl J Med 2021;384:693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wong G‐H, Yuen B‐Y, Chan H‐Y, Tse Y‐K, Yip T‐F, Lam K‐Y, et al. Impact of dose and duration of corticosteroid on the risk of hepatitis flare in patients with chronic hepatitis B. Liver Int 2019;39:271‐279. [DOI] [PubMed] [Google Scholar]
- 13. Wong G‐H, Wong V‐S, Yuen B‐Y, Tse Y‐K, Yip T‐F, Luk H‐S, et al. Risk of hepatitis B surface antigen seroreversion after corticosteroid treatment in patients with previous hepatitis B virus exposure. J Hepatol 2020;72:57‐66. [DOI] [PubMed] [Google Scholar]
- 14. Hospital Authority . Hospital authority statistical report 2018‐2019. https://www3.ha.org.hk/data/HAStatistics/StatisticalReport/2018‐2019. Accessed April 29, 2020.
- 15. Wong JC, Chan HL, Tse YK, Yip TC, Wong VW, Wong GL. Statins reduce the risk of liver decompensation and death in chronic viral hepatitis: a propensity score weighted landmark analysis. Aliment Pharmacol Ther 2017;46:1001‐1010. [DOI] [PubMed] [Google Scholar]
- 16. Yip TC, Wong VW, Chan HL, Tse YK, Lui GC, Wong GL. Tenofovir is associated with lower risk of hepatocellular carcinoma than entecavir in patients with chronic HBV infection in China. Gastroenterology 2020;158:215‐225.e6. [DOI] [PubMed] [Google Scholar]
- 17. Lai J‐T, Wong G‐H, Yip T‐F, Tse Y‐K, Lam K‐Y, Lui G‐Y, et al. Chronic hepatitis B increases liver‐related mortality of patients with acute hepatitis E: a territorywide cohort study from 2000 to 2016. Clin Infect Dis 2018;67:1278‐1284. [DOI] [PubMed] [Google Scholar]
- 18. Lui GCY, Wong NS, Wong RYK, Tse YK, Wong VWS, Leung CC, et al. Antiviral therapy for hepatitis B prevents liver injury in patients with tuberculosis and hepatitis B coinfection. Clin Infect Dis 2020;70:660‐666. [DOI] [PubMed] [Google Scholar]
- 19. Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HLY, Chen CJ, et al. Asian‐Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American DA. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018;41(Suppl.):S13‐S27. [DOI] [PubMed] [Google Scholar]
- 21. Braitman LE, Rosenbaum PR. Rare outcomes, common treatments: analytic strategies using propensity scores. Ann Intern Med 2002;137:693‐695. [DOI] [PubMed] [Google Scholar]
- 22. Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 2006;98:253‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med 2013;32:3388‐3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Friedman JH. Greedy function approximation: a gradient boosting machine. Ann Stat 2001;29:1189‐1232. [Google Scholar]
- 25. McCaffrey DF, Ridgeway G, Morral AR. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods 2004;9:403‐425. [DOI] [PubMed] [Google Scholar]
- 26. Stuart EA, Lee BK, Leacy FP. Prognostic score–based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol 2013;66(Suppl.):S84‐S90.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med 2009;28:3083‐3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hamilton BH, Ko CY, Richards K, Hall BL. Missing data in the American College of Surgeons National Surgical Quality Improvement Program are not missing at random: implications and potential impact on quality assessments. J Am Coll Surg 2010;210:125‐139.e2. [DOI] [PubMed] [Google Scholar]
- 29. Little RJ, D'Agostino R, Cohen ML, Dickersin K, Emerson SS, Farrar JT, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med 2012;367:1355‐1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med 2009;28:1982‐1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Austin PC. The use of propensity score methods with survival or time‐to‐event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014;33:1242‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rubin DB, Schenker N. Multiple imputation in health‐care databases: an overview and some applications. Stat Med 1991;10:585‐598. [DOI] [PubMed] [Google Scholar]
- 33. European Association for the Study of the Liver . Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017;2017:370‐398. [DOI] [PubMed] [Google Scholar]
- 34. Terrault NA, Lok ASF, McMahon BJ, Chang K‐M, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao J, Cui W, Tian BP. Efficacy of tocilizumab treatment in severely ill COVID‐19 patients. Crit Care 2020;24:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck‐Ytter YT; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148:215‐219, quiz e16‐7. [DOI] [PubMed] [Google Scholar]
- 37. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Drug‐induced liver injury. J Hepatol 2019;70:1222‐1261. [DOI] [PubMed] [Google Scholar]
- 38. Devarbhavi H. An update on drug‐induced liver injury. J Clin Exp Hepatol 2012;2:247‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sagir A, Erhardt A, Schmitt M, Haussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology 2008;47:592‐595. [DOI] [PubMed] [Google Scholar]
- 40. Patel K, Sebastiani G. Limitations of non‐invasive tests for assessment of liver fibrosis. JHEP Rep 2020;2:100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mapakshi S, Kramer JR, Richardson P, El‐Serag HB, Kanwal F. Positive predictive value of International Classification of Diseases, 10th revision, codes for cirrhosis and its related complications. Clin Gastroenterol Hepatol 2018;16:1677‐1678. [DOI] [PubMed] [Google Scholar]
- 42. Wong G‐H, Chan H‐Y, Loo C‐K, Hui Y‐T, Fung J‐Y, Cheung D, et al. Change in treatment paradigm in people who previously injected drugs with chronic hepatitis C in the era of direct‐acting antiviral therapy. J Gastroenterol Hepatol 2019;34:1641‐1647. [DOI] [PubMed] [Google Scholar]
- 43. Wong GL, Hui VW, Yip TC, Tse YK, Yuen PC, Wong VW. Secular trend of disease burden in patients with chronic viral hepatitis—a territory‐wide study of 143,701 patients with data from HADCL in 2000‐2018. Hepatol Int 2021;15:A322. [Google Scholar]
- 44. Hui YT, Wong GLH, Fung JYY, Chan HLY, Leung NWY, Liu SD, et al. Territory‐wide population‐based study of chronic hepatitis C infection and implications for hepatitis elimination in Hong Kong. Liver Int 2018;38:1911‐1919. [DOI] [PubMed] [Google Scholar]
- 45. Butt AA, Yan P, Chotani RA, Shaikh OS. Mortality is not increased in SARS‐CoV‐2 infected persons with hepatitis C virus infection. Liver Int 2021. Feb 3. 10.1111/liv.14804. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


