Figure 1. Flow of Participants in Study, Interventions, and Overall Study Design.
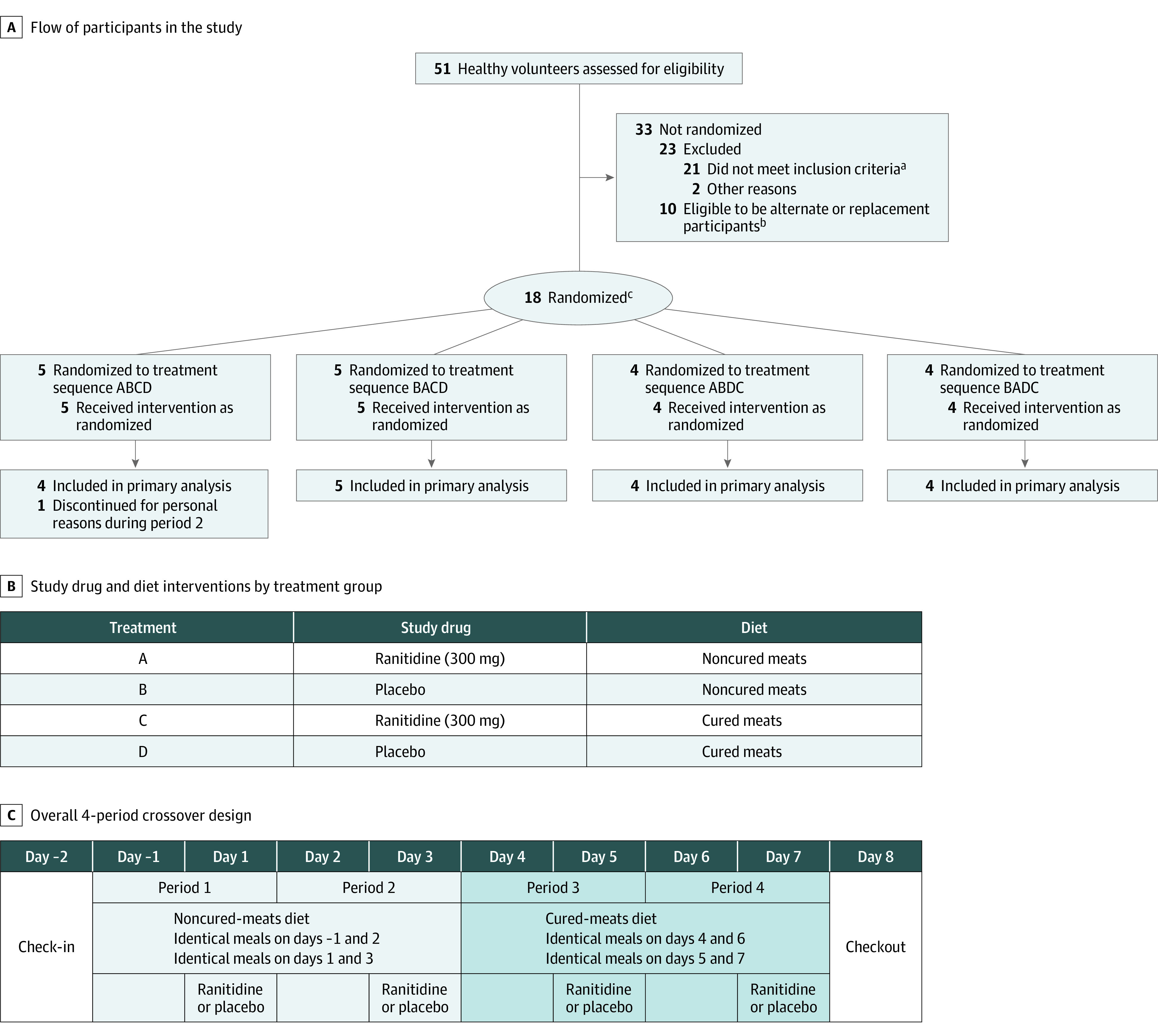
aThe most common reason for participant exclusion was a history of Helicobacter pylori infection or ulcer disease or a positive H pylori breath test result (n = 12). Additional reasons for exclusion: abnormal medical history findings, clinical laboratory results, vital sign measurements, 12-lead electrocardiogram results, or physical examination findings at screening (n = 5); having signs or symptoms consistent with COVID-19 (n = 3); nicotine use within 6 weeks of screening (n = 1); history of clinically significant disorder, condition, or disease (n = 1); or clinically significant laboratory results (n = 1).
bA sample size of 14 participants was determined to have greater than 90% power to detect an increase in 24-hour urinary excretion of N-nitrosodimethylamine (NDMA). Eighteen participants were randomized in a single cohort to account for potential dropouts without needing to enroll an additional cohort. This was done so all participants would be served identical meals with the same sourcing of fresh foods to avoid potential variability in nitrite, nitrate, or NDMA content. Because there was a delay between screening and determining final eligibility for the study (eg, receiving all laboratory tests), 10 additional people met all inclusion criteria and were eligible to be alternates (eg, if someone had to cancel or did not show up at check-in) or replacements (eg, if there had been fewer than 14 completers and a second cohort was enrolled).
cRandomization was conducted in block sizes of 4. The remaining 2 participants were randomly placed in 2 of the 4 treatment sequences.
