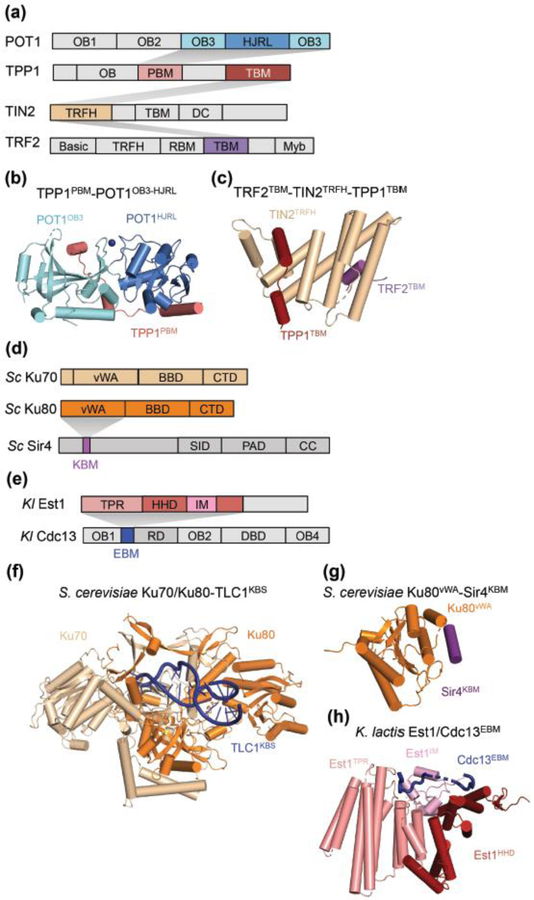
Figure 4.
Structures of protein domains and sub-complexes with important roles in telomerase recruitment to telomeres. Structures recently determined are shown in colors. The shaded wedges indicate interactions. (a) Domain architectures of human POT1, TPP1, TIN2 and TRF2. OB indicates OB-folds. POT1 has three OB-fold domains and a Holliday junction resolvase-like (HJRL) domain embedded within the third OB-fold. TPP1 has an OB-fold domain, a POT1-binding motif (PBM), and a TIN2-binding motif (TBM). TIN2 has a TRFH domain, a TRFH-binding motif (TBM) and a dyskeratosis congenita (DC) disease mutation hotspot region. TRF2 has a basic domain, a TRFH domain, a RAP1-binding motif (RBM), a TIN2-binding motif (TBM) and a Myb DNA-binding domain. (b) Structure of the third OB-fold (OB3) and HJRL domains of POT1 in complex with the PBM of TPP1 (PDB 5H65) [56,57]. (c) Structure of the TRFH domain of TIN2 in complex with the TBMs of TPP1 and TRF2 (PDB 5XYF) [58]. (d) Domain architecture of S. cerevisiae Ku70, Ku80 and Sir4. Each of Ku70 and Ku80 contains a von Willebrand factor type A domain (vWA), a β-barrel domain (BBD) and a C-terminal α-helical domain (CTD). Sir4 has a Ku-binding motif (KBM), a Sir2-interacting domain (SID), a partitioning and anchoring domain (PAD), and a coiled-coil (CC) domain. (e) Domain architecture of K. lactis Est1 and Cdc13. Est1 has a tetratricopeptide repeat domain (TPR), a helical hairpin domain (HHD), and an insertion motif (IM). Cdc13 has three OB-fold domains, OB1, OB2 and OB4, an Est1-binding motif (EBM), a recruitment domain (RD), and a DNA-binding domain (DBD). (f) Structure of S. cerevisiae Ku70/Ku80 and the Ku binding site of TLC1 (PDB 5Y58) [25]. (g) Structure of S. cerevisiae Ku80 vWA domain with Sir4 KBM (PDB 5Y59) [25]. (h) Structure of K. lactis Est1 in complex with EBM of Cdc13 (PDB 5Y5A) [25].