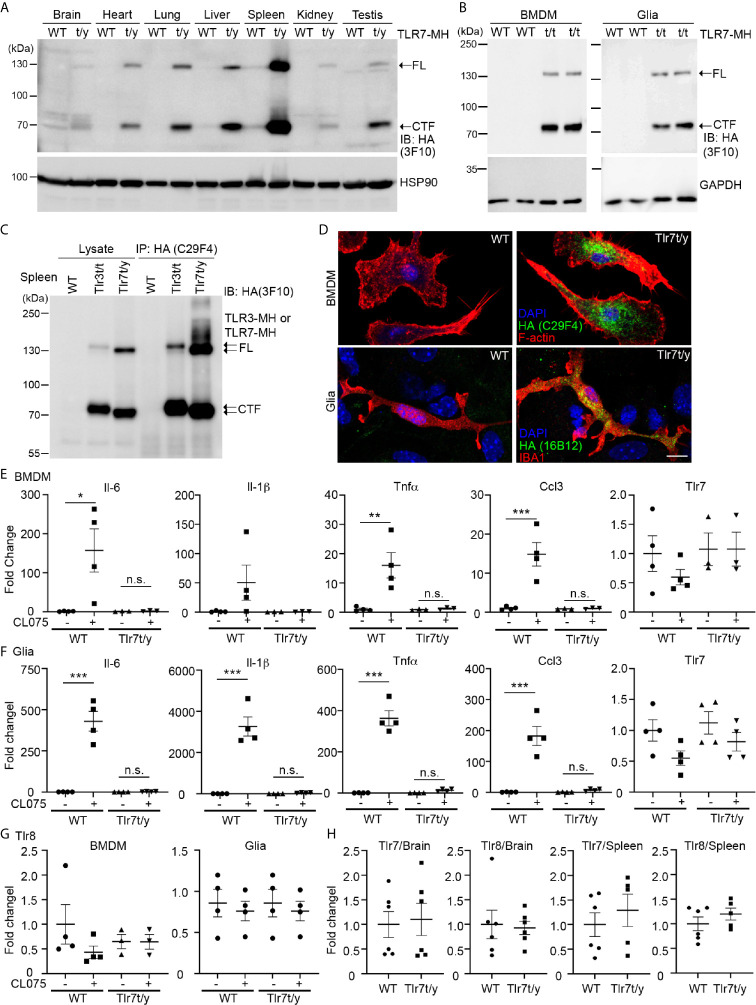
Figure 6.
C-terminal Myc-HA dual tagging results in inactivation of TLR7. (A, B) Immunoblotting using anti-HA antibodies was performed to detect the expression of TLR7-MH in Tlr7t/y tissues (A) and Tlr7t/t cells (B). Different organs and BMDMs and glia cell cultures were examined, as indicated. Samples prepared from WT littermates were used as negative controls. HSP90 and GAPDH were used as a loading control. FL, full-length TLR7-MH; CTF, C-terminal fragment of TLR7-MH. (C) Distinct levels of proteolyzed TLR3-MH and TLR7-MH in mouse spleen. Anti-HA antibody (C29F4) was used to precipitate TLR3-MH and TLR7-MH proteins from Tlr3t/t and Tlr7t/y mouse spleens, respectively. WT mice were used as a negative control. The IP complex was then subjected to IB analysis using anti-HA antibody (3F10). FL: full-length TLR3-MH or TLR7-MH, CTF: C-terminal fragment of TLR3-MH or TLR7-MH. (D) Immunostaining of BMDMs and glial cells using anti-HA and anti-IBA1 (for microglia) antibodies was performed, as indicated. Phalloidin and DAPI were used to label F-actin and nuclei, respectively. Scale bars, 10 μm. (E–G) BMDMs and glia cells prepared from WT and Tlr7t/t mice were treated with 4 μM CL075, a TLR7 agonist, for 6 h. Expression levels of Il-6, Il-1b, Tnfa, Ccl3, Tlr7 and Tlr8 were then determined using quantitative RT-PCR and normalized against the internal control Hprt. (H) Levels of Tlr7 and Tlr8 mRNA are similar between WT and Tlr7t/y mouse organs. Brain and spleen of WT and Tlr7t/y mice were harvested for RNA extraction and then subjected to quantitative RT-PCR to analyze levels of Tlr7 and Tlr8, normalized against the internal control Hprt. Data are represented as mean ± SEM (error bars). Each dot indicates the result of one independent culture. *p > 0.05; **p > 0.01; ***p > 0.001; n.s., non-significant. (E–G) Two-way with Bonferroni’s multiple comparisons test.