Figure 4.
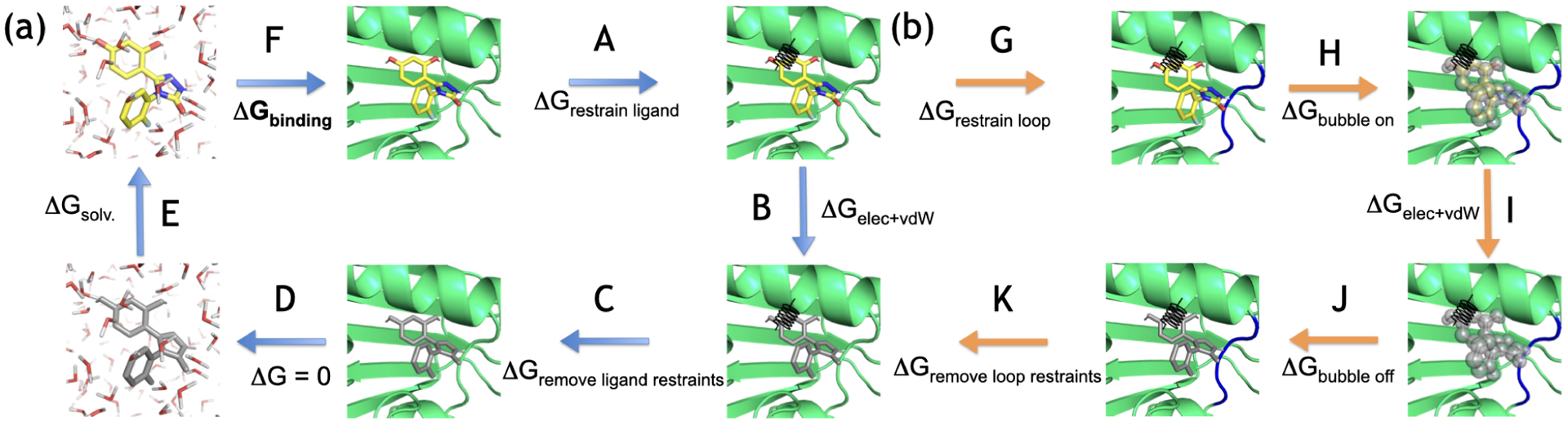
Thermodynamic cycle for computing binding free energies. The left part of the cycle (a) represents the standard cycle used in this paper, the right part (b) was only added for calculations including a bubble-ligand (Sec. 5.5.3). The ligand is restrained using orientational (Boresch-style)52 restraints (A) and then decoupled in the binding site, meaning that the intermolecular electrostatic and van der Waals (vdW) interactions of the ligand are turned off (B). The restraints are released analytically (C), the ligand is transferred from the complex into the solvent (D) and then coupled in the solvent (E). The next leg (F) represents the free energy difference upon ligand binding which is obtained through summation along the cycle. (b) For calculations that include a bubble-ligand to overcome insufficiencies in water sampling, a loop and three buried water molecules are restrained (G, Sec. 5.5.3), then the vdW interactions between the bubble-ligand and water are turned on (H). In the next step the ligand is decoupled in the binding site (I) and the bubble-ligand prevents water from entering the site. The bubble-ligand is then turned off again (J), the restraints on the loop and buried water molecules are released (K) which leads back to the regular cycle.
