Figure 2.
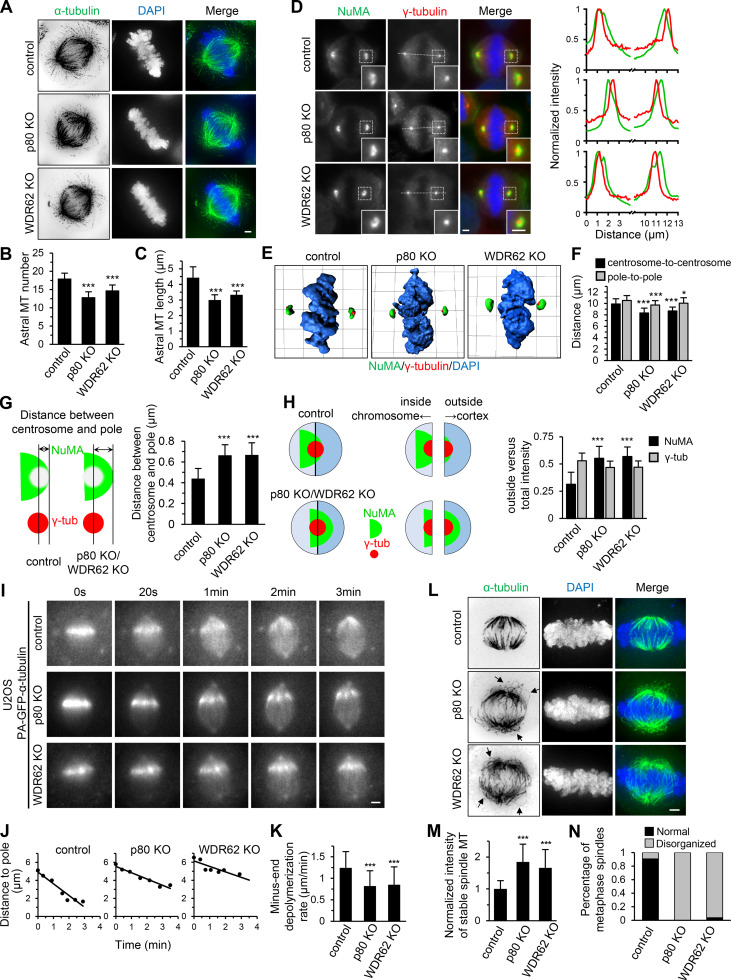
WDR62-katanin regulates spindle organization and dynamics.(A) Immunofluorescence staining for α-tubulin and DAPI in control and indicated knockout (KO) HeLa cells. Maximum intensity projections of Z series with 30 stacks at 0.11-µm steps are shown. (B and C) Quantification of astral MT number and length as shown in A. n = 28, 24, and 20 spindle poles for control, p80 knockout, and WDR62 knockout, respectively. (D) Left: Immunofluorescence staining for NuMA, γ-tubulin, and DAPI in control and the indicated knockout HeLa cells. Insets show enlargements of the boxed areas. Right: Normalized line-scan intensity profiles of NuMA (green) and γ-tubulin (red) corresponding to the white dashed lines in left images. (E) 3D reconstruction of images shown in D. The length of the grid in the images is 3.65 µm. (F) Quantification of centrosome-to-centrosome distance and pole-to-pole distance (see Materials and methods for details) in control and indicated knockout HeLa cells. n = 40 cells for all conditions. (G) Left: Schematic representation of the distance between centrosome and pole (denoted by double-headed arrow), as measured from the center of γ-tubulin (γ-tub) spot (red) to the distal edge of NuMA spot (green). Right: Quantification of the distance between centrosome and pole in control and indicated knockout HeLa cells. n = 80 poles for all conditions. (H) Left: Schematic representation of outside and inside. The centers of two semicircular ROIs were selected to overlap with the visually determined center of the γ-tubulin spot (red). The chromosome- and cortex-directed ROIs were referred to as inside and outside, respectively. Right: Quantification of outside versus total intensity of γ-tubulin and NuMA in control and indicated knockout HeLa cells. n = 80 poles for all conditions. (I and J) Time-lapse images of PA-GFP-tubulin stripe in control or indicated knockout U2OS cells stably expressing PA-GFP–α-tubulin (I) and corresponding plots of the distance between PA-GFP stripe and spindle pole against time (J). (K) Quantification of minus-end depolymerization rate in control and indicated knockout U2OS cells. The minus-end depolymerization rate was derived from the plot slopes in J. Control, n = 33 cells; p80 knockout, n = 22; WDR62 knockout, n = 27. (L) Following cold treatment at 0°C for 5 min, the control and indicated knockout HeLa cells were fixed and immunostained for α-tubulin and DAPI. Maximum intensity projections of Z series with 30 stacks at 0.11-µm steps are shown. Arrows denote the curved spindle MTs. (M) Quantification of integrated intensity of spindle MTs in control and indicated knockout HeLa cells as shown in L. The intensity of each spindle was averaged from four middle stacks. n = 20 cells for all conditions. (N) Quantification of percentage of metaphase cells with normal or disorganized spindles in control and indicated knockout HeLa cells as shown in L. From left to right, n = 314, 280, 306 cells. Scale bars, 2 µm. Data represent mean ± SD. *, P < 0.05; ***, P < 0.001; two-tailed t test.