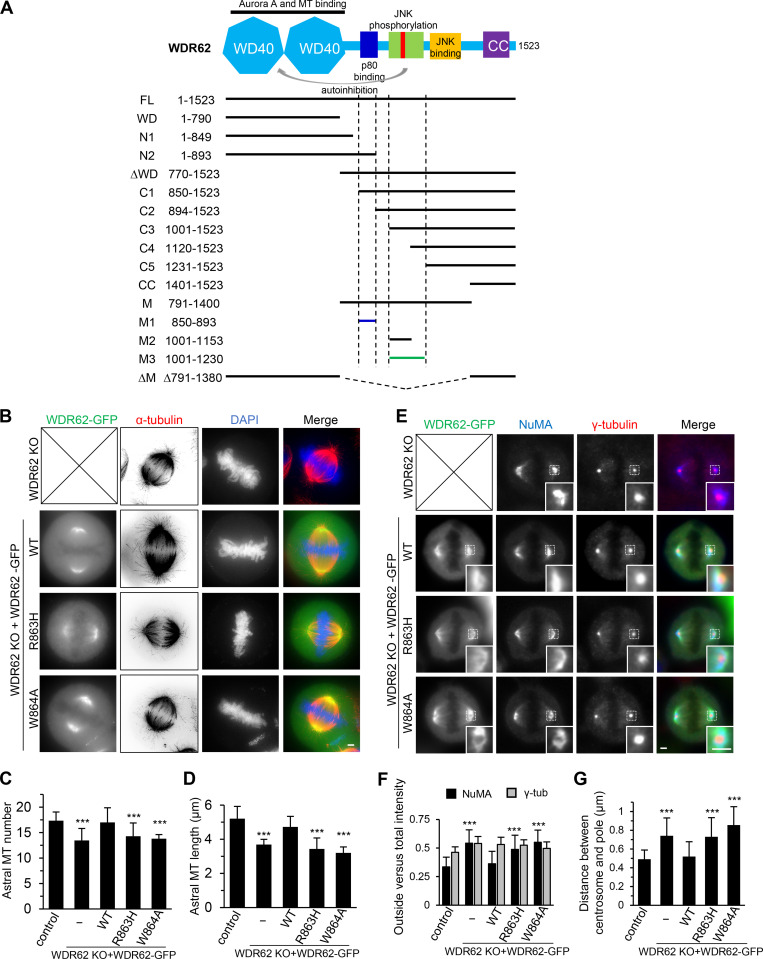
Figure S2.
Overview of WDR62 deletion mutants used in this study and the rescue of spindle defects by WT WDR62-GFP but not its katanin-binding deficient mutants.(A) Schematic illustration of the functional domains of WDR62 and summary of all the deletion mutants used in this study. WD40 domain (WD; light blue): MT binding and Aurora A binding; M1 (dark blue): p80 binding; M3: (green): autoinhibitory domain that interacts with WD40 domain. The horizontal lines were drawn to indicate the region spanned by the corresponding fragments (M1: dark blue; M3: green; others: black). The vertical dashed lines were drawn to indicate the boundaries of fragments M1 and M3. Note that JNK phosphorylation site T1053 (red) was located within the autoinhibitory M3 fragment. M, middle region; CC, coiled-coil. (B) Immunofluorescence staining of α-tubulin and DAPI in WDR62 knockout (KO) HeLa cells or the knockout cells transiently transfected with GFP-tagged WT WDR62 or its indicated mutants. Maximum intensity projections of Z series with 30 stacks at 0.11-µm steps are shown. (C and D) Quantification of the number and length of astral MTs shown in B. From left to right, n = 20, 20, 22, 20, and 20 spindle poles. (E) Immunofluorescence staining of NuMA and γ-tubulin in WDR62 knockout HeLa cells or the knockout cells transiently transfected with GFP-tagged WT WDR62 or its indicated mutants. Insets show enlargement of boxed areas. (F and G) Quantification of “outside” versus total intensity of γ-tubulin (γ-tub) and NuMA (F) and distance between the centrosome and the pole (G) shown in E. n = 50 spindle poles for all conditions in F; n = 48 spindle poles for all conditions in G. Scale bars, 2 µm. Data represent mean ± SD. ***, P < 0.001; two-tailed t test.