Figure 2.
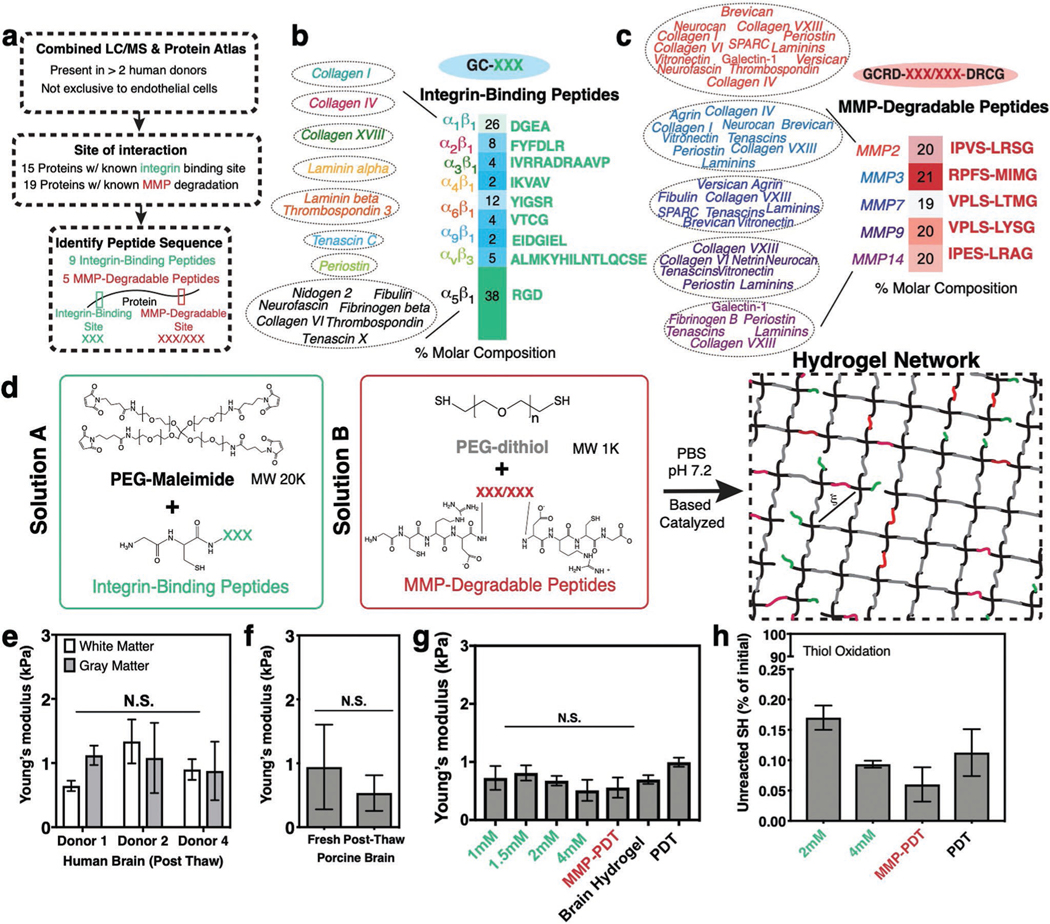
Design of a synthetic brain ECM hydrogel. a) Proteins identified via in-house proteomics (LC/MS, present in >2 donors) and histology from the Protein Atlas (to exclude proteins specific to endothelial cells) were screened by integrin-binding and matrix metalloproteinase (MMP)-degradable peptide sequences. b) Integrin-binding peptides include 15 proteins, incorporated into the hydrogel via single cysteine. Heat map depicts molar concentration of each peptide in the final design. c) MMP-degradable peptides include 19 proteins, and were incorporated into the hydrogel by a cysteine at each end. Heat map depicts molar concentration of each peptide in the final design. d) A Michael-addition reaction is used to combine these peptides with PEG-maleimide to form a hydrogel network via two solutions: solution A containing integrin-binding peptides and the PEG polymer, and solution B with the MMP-degradable peptides and a nondegradable crosslinker. ξ is the mesh size of the hydrogel (≈20 nm). e) Young’s modulus of previously frozen human brain (3 donors), f) fresh and previously frozen porcine brain n = 6, and g) hydrogel tuned to the same Young’s modulus as brain tissue as measured via indentation. Modulus is maintained after incorporation of integrin-binding (green) or MMP-degradable (red) peptides. n = 8. h) Percent of unreacted thiols after hydrogel is formed, and then reduced with NaBH4, indicating high network formation efficiency. n = 8. All data are mean ± s.d. Data in (e)–(g) were analyzed using an ANOVA followed by a Tukey’s multiple comparison test with 95% confidence interval. N.S. is not significant.