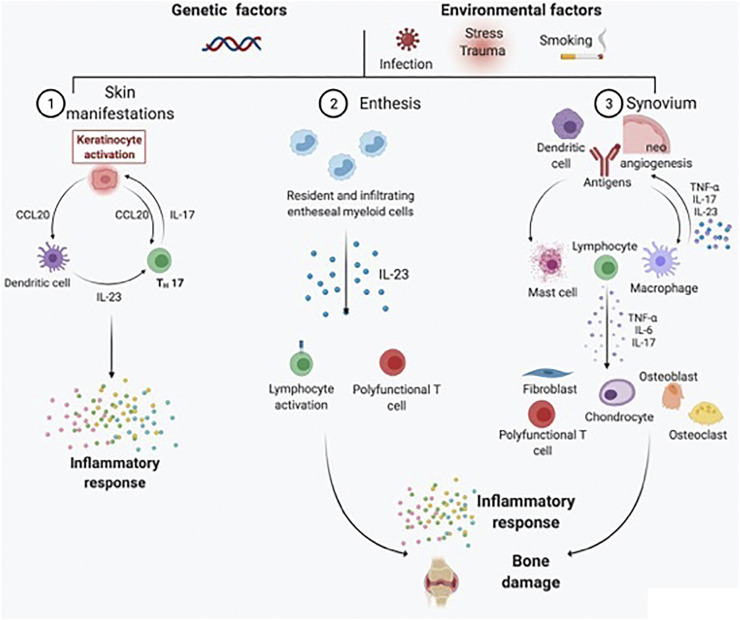
FIGURE 1.
Schematic representation of the main pathogenic processes driving PsA development and chronicity. PsA is a heterogeneous chronic disease, with skin, entheseal, and synovial tissue involvement occurring in different proportions across patients under genetic and environmental triggers. For skin manifestations, activated dendritic cells secrete predominantly IL-23, which in turn induces the differentiation of naïve T cells into Th17 cells. IL-17 is responsible for keratinocyte activation and subsequent perpetuation of skin inflammation. At the entheseal level, resident and infiltrating entheseal myeloid cells produce IL-23, which is responsible for lymphocyte activation and the inflammatory response, as well as bone damage. Psoriatic synovitis, on the contrary, is characterized by tortuous and immature neoangiogenesis, with antigen presentation by dendritic cells and macrophages leading to lymphocyte activation. As a result, the synovial inflammatory infiltrate is rich in activated lymphocytes, mast cells, and macrophages. Polyfunctional T cells are responsible for the production of several types of proinflammatory cytokines (e.g., TNF-α, IL-17A, GM-CSF, and IFN-γ) in the synovial membrane and synovial fluid. The proinflammatory cytokine milieu further activates fibroblast-like synoviocytes, chondrocytes, osteoblasts, and osteoclasts, resulting in bone damage.