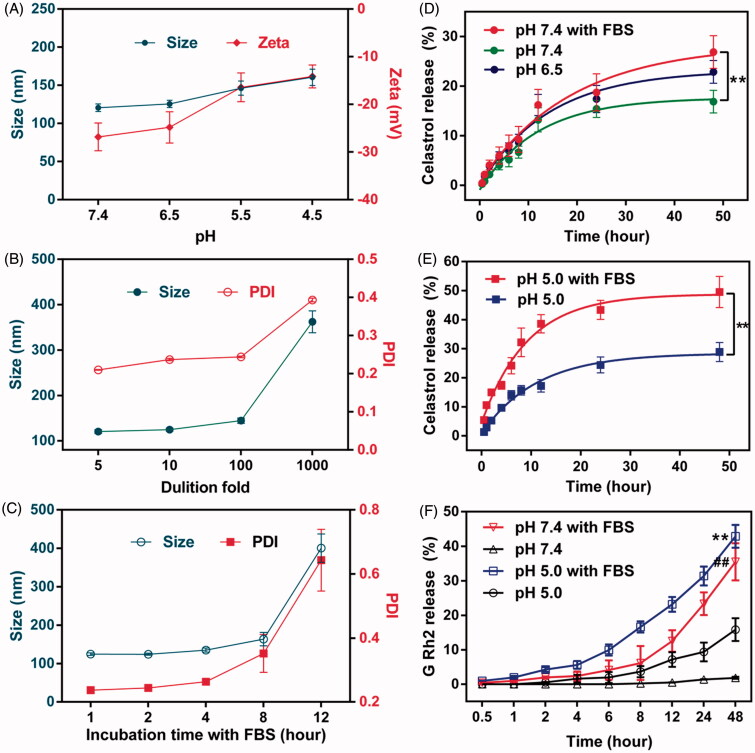
Figure 4.
Evaluation on stability and drug release. (A) The changes in the particle size and zeta potential of CG-M under different pH environments, (B) the changes in the particle size and PDI of CG-M under various dilutions, (C) the changes in the particle size and PDI of CG-M after incubation with the 50% (v%) FBS for 12 h, (D) accumulative release rate of celastrol from CG-M at pH 7.4, pH 7.4 + 50% FBS and pH 6.5 within 48 h, (E) accumulative release rate of celastrol from CG-M at simulated endosomal environment within 48 h and (F) accumulative release rate of G Rh2 from CG-Mblank at various pH and simulated physiologic environments within 48 h. **p < 0.01 versus pH 5.0; ##p < 0.01 versus pH 7.4. All the data are presented as mean ± SD (n = 3).