Abstract
The COVID-19 pandemic has a major impact on a wide range of health outcomes. Disruptions of elective health services related to cervical screening, management of abnormal screening test results, and treatment of precancers, may lead to increases in cervical cancer incidence and exacerbate existing health disparities. Modeling studies suggest that a short delay of cervical screening in subjects with previously negative HPV results has minor effects on cancer outcomes, while delay of management and treatment can lead to larger increases in cervical cancer. Several approaches can mitigate the effects of disruption of cervical screening and management. HPV-based screening has higher accuracy compared to cytology, and a negative HPV result provides longer reassurance against cervical cancer; further, HPV testing can be conducted from self-collected specimens. Self-collection expands the reach of screening to underserved populations who currently do not participate in screening. Self-collection and can also provide alternative screening approaches during the pandemic because testing can be supported by telehealth and specimens collected in the home, substantially reducing patient-provider contact and risk of COVID-19 exposure, and also expanding the reach of catch-up services to address backlogs of screening tests that accumulated during the pandemic. Risk-based management allows prioritizing management of patients at highest risk of cervical cancer while extending screening intervals for those at lowest risk. The pandemic provides important lessons for how to make cervical screening more resilient to disruptions and how to reduce cervical cancer disparities that may be exacerbated due to disruptions of health services.
Keywords: Cervical cancer, Cervical precancer, Screening, HPV, COVID-19, SARS-CoV-2, Pandemic, Health disparities
1. Cervical cancer prevention approaches
Almost all cervical cancers are caused by persistent infections with one of 12–14 carcinogenic human papillomavirus (HPV) types (Schiffman et al., 2016). While most HPV infections become undetectable after few months, some may persist and progress to cervical precancer. A subset of precancers will progress to invasive cancer if left untreated. Understanding the role of HPV in cervical carcinogenesis has led to development of important HPV-based prevention approaches. HPV vaccines were first introduced in the US in 2006. HPV vaccines should ideally be administered before onset of sexual activity to maximize the preventive effects. First generation vaccines targeted only the two most carcinogenic types, HPV16 and HPV18, which are responsible for about 70% of cervical cancers. In 2014, a nonavalent HPV vaccine was introduced covering seven carcinogenic HPV genotypes (HPV16, 18, 31, 33, 45, 52, 58), potentially preventing over 90% of cervical cancers. Vaccination is routinely recommended for ages 11–12, but may begin at age 9, and extended age ranges for catch-up vaccination have been approved (Meites et al., 2019). Birth cohorts who received routine HPV vaccinations at ages 11–12 only recently entered the age groups at which cervical screening is recommended. Studies have shown a strong decrease in HPV prevalence, specific to the types targeted by the vaccines, and to a lesser extent, of types partially covered through cross-protection in women who received the HPV vaccine. Additionally, reduction of vaccine types in unvaccinated women is suggestive of herd protection (Oliver et al., 2017). The first population-based studies have now shown reduced cervical cancer incidence among younger vaccinated women, but it will take decades for the full effect of vaccination to be realized over a wide age range of the screening population (Lei et al., 2020).
Therefore, screening continues to be an essential component of cervical cancer prevention. Successful secondary prevention of cervical cancer relies on a multi-step process, starting with screening of the target population, triage of screen-positives, colposcopy-biopsy to confirm the presence of cervical precancer, and treatment of precancers. Cervical screening using the Pap test, or cervical cytology, for decades had major impact on reducing cervical cancer incidence in countries with broad population coverage (Schiffman et al., 2016). However, cervical cytology suffers from low sensitivity and low reproducibility. HPV testing first received regulatory approval as a triage test for minimally abnormal cytology results (2000), then as a screening co-test with cytology (2003), and more recently as a stand-alone test (2014) (Food and Drug Administration, 2019). HPV-based screening has higher sensitivity and reproducibility compared to cytology, making cervical screening more efficient (Kim et al., 2018; Melnikow et al., 2018). Currently, guidelines recommend three strategies for cervical screening: cytology alone, HPV-cytology co-testing, and HPV testing alone (Curry et al., 2018; Fontham et al., 2020). Women testing positive for HPV require additional triage tests to decide who needs to be referred for colposcopy and biopsy evaluation (Wentzensen et al., 2016). In the US, currently approved triage strategies include cytology, partial genotyping, extended genotyping, and dual stain cytology. With some exceptions, colposcopy with multiple biopsies is typically recommended for confirmation of cervical precancer before treatment (Wentzensen et al., 2015; Wentzensen et al., 2017).
Multi-step cervical cancer screening programs allow identification of the approximately 1% of women at highest risk of precancer who need treatment, while avoiding overtreatment of the majority of women who are at lower risk. However, a downside of multi-step programs is the need for multiple patient visits, including screening, colposcopy, treatment, and surveillance. At each step, there is a risk of loss to follow-up due to patient-, provider-, or health system-related factors, and an untreated precancer may progress to cancer.
2. Cervical cancer disparities
2.1. Populations affected by cervical cancer disparities
Despite overall declines in cervical cancer incidence and mortality in the U.S., over ten thousand cervical cancer cases and four thousand deaths still occur each year, with significant disparities observed by race and ethnicity, geographic region, and socioeconomic status (Collins et al., 2014; Downs et al., 2008). Improved access to cervical screening of underserved populations has somewhat reduced these disparities over time; however, they remain pervasive among many groups. To accurately compare cervical cancer incidence and mortality rates between populations, it is important to account for hysterectomy, which is very common in the U.S. and varies significantly by race, ethnicity, and geographic region (e.g., more common in Blacks and in the Southern U.S.) (Clarke et al., 2019). Hysterectomy-corrected analyses have revealed racial and ethnic disparities in cervical cancer incidence and mortality, showing higher incidence in non-Hispanic Blacks (9.0 per 100,000) and Hispanics (7.6 per 100,000) compared to non-Hispanic Whites (5.4 per 100,000) and Asian/Pacific Islanders (4.5 per 100,000) from 1999 to 2015 (Islami et al., 2019) and higher corrected mortality in Blacks (10.1 per 100,000) compared to Whites (4.7 per 100,000) from 2000 to 2012 (Beavis et al., 2017). In addition to having higher incidence and mortality rates, Non-Hispanic Blacks and Hispanics are also less likely to be diagnosed with localized disease and have worse survival compared to their non-Hispanic White and Asian/Pacific Islander counterparts (Coker et al., 2009; Eggleston et al., 2006; Islami et al., 2019). Higher cervical cancer incidence and mortality have also been reported among American Indian and Alaskan Native populations (U.S. Department of Health and Human Services, 2020), as well as Vietnamese Americans (Miller et al., 2008).
Regional differences in cervical cancer incidence and mortality have also been observed. For example, women in rural and nonmetropolitan areas have higher incidence and mortality rates compared to those living in metropolitan areas, and those living in the South have higher incidence and mortality compared to other U.S. regions (Henley et al., 2017; Lulu Yu and Mary, 2019; Singh, 2012). Evidence suggests that some observed racial/ethnic health disparities may be tied to specific geographic regions (Caldwell et al., 2016; Lulu Yu and Mary, 2019; Probst et al., 2004; Semrad et al., 2011; Yoo et al., 2017). Such examples include Blacks living in the South, Hispanic women living along the Texas-Mexico border (Fernandez et al., 2014), Whites living in Appalachia (Wilson et al., 2016), and American Indians and Alaskan Natives living in the Northern Plains (Espey et al., 2005).
Many of the observed racial/ethnic and regional disparities are linked to social determinants of health such as poverty, lack of insurance coverage, and poor access to care. Underlying factors that affect healthcare access include misconceptions about risk of cervical cancer and the importance of screening among patients, frequent changing of screening guidelines creating confusion among patients and providers, patient fear and mistrust of the healthcare system, different cultural beliefs, transportation issues, and lack of insurance (Freeman, 2005; Nolan et al., 2014). Moreover, healthcare providers' unconscious bias and racism have been shown to negatively impact Black patients' receipt of screening and follow-up care (Nolan et al., 2014). In many settings, lack of healthcare providers remains a major challenge. As of 2010, 49% of US counties lacked an obstetrician-gynecologist, and most of these counties were in rural areas (ACOG Committee Opinion No. 586, 2014). Black and Hispanic individuals are also more likely to live in communities without access to an obstetrician-gynecologist (Hung et al., 2017). A recent meta-analysis summarized multiple factors contributing to reduced cervical cancer screening access in rural areas, including limited availability of healthcare providers qualified for cervical screening, high clinician turnover, long waiting periods, inflexible clinic hours, and lack of transportation among others (Majid et al., 2019).
Comorbid conditions, such as obesity, are more common in minority populations and can adversely affect cervical cancer outcomes. Obesity has been associated with increased cervical cancer incidence and mortality (Bhaskaran et al., 2014; Calle et al., 2003; Clarke et al., 2018). Obese patients are less likely to participate in cervical cancer screening compared to normal weight patients, and are also less likely to be offered cervical cancer screening by providers (Adams et al., 1993; Maruthur et al., 2009; Wee et al., 2000). Providers have reported that cervical cancer screening and management are more challenging in obese compared to normal weight patients (Clarke et al., 2020), and epidemiologic data from a large well-screened population suggest that up to 20% of cervical cancers diagnosed in obese women may be attributed to missed detection and treatment of cervical precancers (Clarke et al., 2018). In this study, there were no differences in stage at diagnosis by body mass index.
2.2. Disparities across the range of cervical cancer prevention
Underlying causes of cervical cancer disparities are multi-factorial and impact all steps of the process from primary and secondary prevention approaches to treatment. A meta-analysis of over three million people in the U.S. showed that racial and ethnic minority adolescents were more likely to initiate the HPV vaccine, but less likely to receive all three doses compared to Whites (Spencer et al., 2019). While higher rates of HPV vaccine initiation were found among adolescents living below the federal poverty level, adolescents living outside of Metropolitan Areas (Walker et al., 2019), and in the Southern U.S. have lower HPV vaccination rates (Centers for Disease Control and Prevention, 2020b). While school-based vaccination programs could address some of these disparities, only few schools have health centers that may provide vaccination and children attending schools with vaccination programs have vaccination rates similar to the general population (Oliver et al., 2019).
Effective secondary prevention of cervical cancer requires population-based screening, timely follow-up of screen-positives, and treatment of precancerous lesions. Failure at any of these steps puts individuals at risk of developing cancer. Lower rates of cervical screening are observed among certain Asian populations, Hispanics, and among foreign-born individuals, particularly those that immigrated to the U.S. within 10 years. Screening rates are also lower in women with lower educational attainment, and those who are uninsured (Saslow et al., 2012). Women in rural areas who may have limited access to healthcare are also less likely to be up-to-date with cervical cancer screening (Datta et al., 2006; Horner-Johnson et al., 2015). Healthcare providers play an important role in overcoming cancer prevention barriers. Lack of language concordance and cultural sensitivity has been noted as a barrier to cervical cancer screening among Black, Hispanic, and rural populations (Majid et al., 2019). Provider competence and sensitivity is of particular importance for sexual minority populations (Watts et al., 2009).
Although differences in screening may account for a large proportion of cervical cancer disparities, differences in receipt of follow-up diagnostic procedures and treatment play an important role as well. For example, while non-Hispanic Blacks have higher cervical cancer incidence and mortality compared to non-Hispanic Whites, several studies have shown similar screening rates between these two groups (Eggleston et al., 2007; Saslow et al., 2012). In data from the Breast Cervical Cancer Early Detection Program, Blacks were less likely to be followed up after abnormal screening results compared to other racial or ethnic groups (Benard et al., 2005). The goal of the National Cancer Institute's Population-based Research to Optimize the Screening PRocess (PROSPR) Network is to evaluate multilevel variation in screening and follow-up for several cancer sites. The PROSPR Network reported that in a multimodal microsimulation study (Rutter et al., 2018), colposcopy delays led to a lower lifetime benefit of screening with 1.4% fewer cancers prevented at about 90 days, and that minority, lower socioeconomic status, and uninsured women are less likely to complete timely follow-up after an abnormal screening result (Doubeni et al., 2018). Differences in insurance coverage may play a major role, particularly among low-income populations who cannot afford to pay out-of-pocket costs. The Affordable Care Act requires that screening for cervical cancer be provided without cost-sharing; however, coverage does not extend to diagnostic follow-up tests resulting from abnormal screening results, or treatment of precancer or cancer. Medicare Part B covers cervical cytology at no cost, but patients have to pay some, or all of the costs associated with diagnostic follow-up after abnormal screening results (U.S. Centers for Medicare & Medicaid Services., 2021).
Disparities in cervical cancer mortality and survival may also be explained by differences in cervical cancer treatment. Limited findings have suggested that Blacks are less likely to receive appropriate treatment for cervical cancer compared to Whites. Reasons for disparities are multi-factorial, and include differences in the prevalence of comorbidities, patient refusal, factors that influence physician recommendations, structural barriers such as insurance coverage, and clinical setting (Shavers and Brown, 2002). Differences in treatment have also been reported for other minorities including Hispanics and American Indians (Gilliland et al., 1998). Racial and ethnic differences in cervical cancer treatment are likely to be exacerbated in rural and other medically underserved and impoverished settings (Freeman, 2005).
3. Impact of COVID-19 on cervical screening and management, and potential widening of disparities
The COVID-19 pandemic has led to a substantial reduction in preventive healthcare, including HPV vaccinations and cervical cancer screenings. Stay-at-home orders or advisories were implemented in a rolling manner throughout the spring, summer, fall and winter of 2020 in response to waves of COVID-19 infections and hospitalizations (Moreland et al., 2020). Preventive services, including adolescent vaccinations and cancer screenings, were frequently curtailed during stay-at-home restrictions. Early reports suggest that HPV vaccinations dropped by >70% in March 2020, and HPV vaccinations remained 25–50% below baseline levels in June, with a cumulative deficit of over 1 million doses (Hart, 2020). Data from the Epic Health Research Network, which includes 60 healthcare organizations representing 306 hospitals in 28 states covering 9.8 million patients, compared cervical cancer screening in January through June of 2020 to average monthly screening numbers in 2017–2019. Cervical cancer screening dropped by 94% following the national emergency declaration, and even after stay-at-home orders were lifted, screening remained 35% below historical averages. An estimated 40,000 cervical cancer screenings were missed within this network between March and June 2020 (Mast and Munoz del Rio, 2020). Very limited data are available to assess the continued impact of COVID-19 on cancer screenings over the fall and winter of 2020. Although COVID-19 infection rates climbed to historically high levels in many parts of the US during these months, elective medical services, including cancer screenings, often remained available. Data from Southern California indicate approximately 25% fewer cervical cancer screenings were performed from June through September 2020 compared to previous years. These data indicate decreased utilization of preventive services, though the relative contributions of limited visit availability and patient reluctance to attend routine medical care during the pandemic are not clearly defined (Miller et al., 2021). Data from federally qualified health center systems indicates that 90% suspended cervical cancer screening at least once during 2020 (Fisher-Borne Prev Med).
At the same time that the healthcare industry has been strained by caring for COVID-19 patients, it has suffered substantial financial losses, particularly due to reduction of elective procedures and routine care. The American Hospital Association estimates the total losses to hospitals and health systems in 2020 exceeded $323.1 billion (Tribble, 2020). Revenue loss has led to reductions in the clinical workforce, which, combined with workplace safety requirements, has led to limited appointment availability for cervical cancer screening. Early in the pandemic, reductions in volume of outpatient and elective procedures resulted in furloughs, layoffs, and closures of medical practices (Rubin, 2020). Approximately 1.5 million healthcare jobs were temporarily terminated in March and April, and while many services have returned, the healthcare industry employed 527,000 fewer people in November 2020 than in February 2020 (Bureau of Labor Statistics, 2021). Many layoffs and furloughs were concentrated among nurses, case workers, and other staff, and less frequently included physicians (Song et al., 2020). Pandemic-related changes led to an estimated loss of approximately 20,000 medical practices across the US (The Physicians Foundation, 2020). Among medical practices that remain open, workplace safety requirements for personal protective equipment and physical distancing limit the numbers of in-person visits that can be offered each day (Centers for Disease Control and Prevention, 2020a). On average during the pandemic, in-person clinic visit volume was reduced by 44% across practices, and 60–80% of tests or procedures were deferred (Song et al., 2020). As of November 2020, clinicians report that patient and procedure volumes remain 30% below pandemic levels, which has led to closure or merger of 7% of medical practices (Primary Care Collaborative, 2020).
Patient fears of presenting for medical care also play a role in the reduction of elective health services. Early in the pandemic, as emergency rooms filled with COVID patients, overall emergency room visits decreased as patients with other conditions remained at home (Franchini et al., 2020). This led to more severe presentations of life-threatening conditions, including cardiac arrests, appendicitis, and cancer (Baldi and Savastano, 2020; Sutcuoglu et al., 2020; Wang et al., 2020). While the impact of patient fears on cervical cancer screenings are not well documented, data on other elective services indicated that approximately half of patients may choose to delay care (Chang et al., 2020). A national survey of 1114 patients indicated that 40% of patients delayed care due to financial barriers including lack of insurance, high co-payments or deductibles, and depletion of health savings accounts (Rubin, 2020). An additional 25% of patients reported staying home “so as not to be a bother” to their physicians as they assumed primary care practices were overwhelmed with COVID patients. Physicians are beginning to see the negative impact of foregoing care. A survey of 580 primary care clinicians conducted in October 2020 found that a majority noted worsened health burdens among their patients due to delaying care (Primary Care Collaborative, 2020). Delays in accessing care may be particularly exacerbated in communities with high burdens of COVID-19.
Individuals who are most affected by the pandemic are likely to be those who already experience social and healthcare-related inequities. These inequities disproportionately affect minority and low-income communities. Like cervical cancer, COVID-19 incidence and mortality are highest among low-income and rural communities, as well as Black, indigenous, and people of color (Moore et al., 2020). Pandemic-related disruptions in preventive services may widen existing cancer disparities (Ku and Brantley, 2020; Singh and Jemal, 2017). For example, poverty has been exacerbated by the pandemic, with 5.7 million more Americans unemployed in December 2020 compared to February 2020 (Bureau of Labor Statistics, 2021). Unemployment rates and income losses have been widespread, and most pronounced among Blacks, Hispanics, and Asians, as well as those with less than a high school degree.
Individuals living in rural communities also face distinct challenges during the COVID-19 pandemic that may put them at increased risk for developing cervical cancer. Rural women were more likely to face financial barriers to gynecologic care prior to the pandemic (ACOG Committee Opinion No. 586, 2014), and the rural unemployment rate in June 2020 remained nearly double that of February 2020 (Housing Assistance Council, 2020). In addition, rural communities were already suffering from hospital closures and lack of gynecologic care access prior to the pandemic, which were compounded by recent COVID-19-related closures and personnel layoffs (Melvin et al., 2020).
Further, comorbidities like obesity, a newly recognized cervical cancer disparity (Clarke et al., 2018), increases the risk of severe illness and death from COVID-19 infection and is more common in Non-Hispanic Black and Hispanic individuals, and rural communities.
4. Impact of delays in cervical screening and management on cervical cancer rates
A major concern regarding delays in cervical screening, management, and treatment is that precancers are not detected, or are not adequately treated, and may progress to invasive cancer. However, it will take some time to directly observe the impact of pandemic-related care disruptions on various health outcomes, including cancer incidence.
In lieu of directly observed data, analyses based on disease models can provide immediate estimates of the impact that COVID-19 has on delays (defined as complete disruption of services) of cervical screening and resulting cervical cancer cases. A comparative model-based analysis using three independent NCI Cancer Intervention and Surveillance Modeling Network (CISNET)-cervical models consistently showed that COVID-19-related disruptions would result in a small net increase in cervical cancer cases by 2027 (Burger et al., 2020). The estimated increase is greater for women screened with cytology alone compared to co-testing. Most of the additional cervical cancer cases would occur due to disruptions in surveillance, colposcopies, or excisional treatment rather than extended intervals in primary screening. According to the models, delays in screening will only have a small effect on stage at cervical cancer diagnosis. The increase in cancer incidence directly relates to the length of the care delays. While a 6-month delay would lead to an increase of 5 cancer cases per 1 million women screened (74% related to delays in post-screening care), a 24-month delay would lead to an increase of 38 cancer cases per 1 million women screening (42% related to delays in post-screening care). These estimates demonstrate that, in the setting of limited in person appointments, providing management and treatment for women with abnormal screening results is a high priority while screening can be safely deferred for several months among women with a previous negative HPV tests or co-tests.
5. Lessons from the pandemic: How to improve resilience and equity in cervical cancer screening
The COVID-19 pandemic has led to delays of care due to disruption of health services, social distancing measures, and financial and social hardships affecting large parts of the population. Underserved populations are disproportionally affected by these factors and it is expected that cervical cancer disparities will be further exacerbated without focused mitigation efforts. Addressing cervical cancer disparities has long been a focus of several areas of research. Some important developments described in the following sections can improve resilience of cervical screening programs and increase health equity. Many of these developments were underway before the pandemic and it is important to continue to pursue and accelerate these efforts because they can immediately improve access to high-quality screening, follow-up, and treatment (Table 1 ).
Table 1.
Opportunities to improve resilience in cervical screening and management with a focus on disruptions caused by the pandemic.
| Measure | General characteristics | Utility during pandemic | Current availability | Requirements |
|---|---|---|---|---|
| HPV screening | Compared to cytology, better reassurance for screen-negatives; allows safely extending intervals | Patients with a history of negative HPV screens can safely delay screening visits for several months | HPV screening is recommended by major guidelines organizations | More widespread adoption of HPV screening is needed for underserved populations |
| Self-sampling for HPV screening | Extends screening coverage; extends range of providers who can offer screening | Reduces need to attend in person clinic visits, reduces need for patient-provider physical contact; can be supported by telehealth | When using PCR-based tests, self-sampling is equivalent to provider sampling; self-sampling is part of some international screening programs | Regulatory approval is needed; implementation will require access to self-sampling kits; systems for returning samples and ensuring follow up after abnormal results |
| Risk-based screening and management | Better allocation of services based on risk; improved detection of precancer while reducing unnecessary colposcopies | Safely reduce clinic visits among lowest risk group; prioritize services for highest risk groups | Risk-based screening and management guidelines have been widely endorsed in the US | Risk-based screening and management needs to be widely adopted; widespread access to health decision support tools will facilitate adoption |
| Telehealth | Extends reach of consultations; increases convenience of clinic visits; increases access to specialty consultation | Allows consultations without COVID exposure; reduces need to attend clinics, patient- provider contact; allows to identify patients who do require in person visits | Telehealth availability is increasing; is currently reimbursed in the US comparable to in-person visits due to special pandemic-related legislation | Coverage of both audio and video visits is necessary to provide equity to those with limited internet access/data plans and limited technology skills; extension of insurance coverage beyond the pandemic will encourage expansion and innovation |
The President's Cancer Panel launched an effort to evaluate the impact of the pandemic on breast, cervical, colorectal, and lung cancer screening. Stakeholder meetings on cervical cancer screening identified several priorities to mitigate the pandemic's effects on cervical screening (President’s Cancer Panel, 2020). A critical tool for rapidly addressing missed cervical cancer screenings will be implementing HPV-based screening and self-sampling, since HPV self-sampling can extend the reach of screening and provides an alternative to clinic or office visits during the pandemic. Risk-based management can be used to prioritize clinical management of individuals at highest risk of cancer, while delaying clinic visits in those at low risk. Developing infrastructure and access to telehealth can improve and extend availability of high-quality cancer screening via HPV self-sampling while reducing clinic volumes and risk of SARS-CoV-2 exposure.
5.1. HPV-based screening and self-sampling
Two approaches to HPV screening are currently recommended in the US, HPV-cytology co-testing and screening using HPV alone (Curry et al., 2018; Fontham et al., 2020). Both co-testing and HPV alone provide greater long-term reassurance against risk of cervical cancer compared to cytology and thus can be used to extend screening intervals in women testing negative, reducing the overall number of in-person interactions during the COVID-19 pandemic and beyond. The reassurance of a negative HPV test is very similar to that of a negative co-test (Gage et al., 2014; Wentzensen and Clarke, 2021). It is important to note that adding cytology to HPV testing leads to a very limited increase of sensitivity at the cost of much higher test positivity which can lead to unnecessary repeat testing, colposcopies, and treatment, especially if clinicians are using older management guidelines.
An important benefit of HPV-alone testing is that, unlike cytology or co-testing, it can be performed on self-collected samples, providing a safe and effective alternative to clinic-based screening. Large meta-analyses have demonstrated similar accuracy of PCR-based HPV testing from self-collected and clinician-collected samples (Arbyn et al., 2018). HPV self-sampling is part of international organized screening programs to increase participation of non-responders (Polman et al., 2019; Saville et al., 2018). Offering self-sampling can ensure the continuation of cervical cancer screening during the COVID-19 pandemic without compromising safety and efficacy. HPV self-sampling also provides a mechanism for clinics to address backlogs of patients needing screening in settings where visit volume may be constrained even after the immediate threat of the pandemic has passed. Moreover, self-sampling has the potential to expand screening coverage to underscreened and unscreened populations that currently, as a result of the pandemic or otherwise, face barriers related to access to care. Expediting introduction of HPV self-sampling in the U.S. is important to avoid widening cancer disparities. While HPV self-sampling could rapidly expand cervical cancer screening without requiring in-person services, it does not address any barriers to screening related to coverage by health insurance or local/federal programs.
HPV-positive individuals represent approximately 10% of the screening population, therefore self-sampling can reduce the number of patients requiring in-person visits by 90% compared to a screening system based on provider-collected specimens. An important focus of research is on finding triage markers that can be evaluated in self-collected specimens. Current triage relies on cytology, which requires a provider-collected specimen. New technologies like host and viral methylation have shown promise as alternative triage strategies that can be tested from self-collected primary screening samples.
Currently, there are notable disparities regarding screening approaches offered to different populations. Black and Hispanic women are more likely than white women to be screened with cervical cytology alone than with HPV alone or co-testing (MacLaughlin et al., 2019). Since a negative cervical cytology result provides less reassurance against the development of cancer than HPV testing or co-testing (Castle et al., 2018), screening needs to be repeated more often to provide equivalent protection (Curry et al., 2018). Thus, screening with cervical cytology alone may increase risk of cancer development when screening is disrupted like during the COVID pandemic. Increased use of HPV testing in screening among patients with limited screening access will help identify patients at risk for cancer who require more frequent screening, allowing scarce resources to be focused on those most at risk.
5.2. Risk-based screening and management
Several new guidelines for cervical cancer screening and management of abnormal results were released between 2018 and 2020, including revised screening guidelines (Curry et al., 2018; Fontham et al., 2020) and updated management guidelines (Perkins et al., 2020). Screening with HPV testing alone was recommended as an option for screening by the US Preventive Services Task Force in 2018 (Curry et al., 2018), and as the preferred option for screening by the American Cancer Society in 2020 (Fontham et al., 2020). The 2019 consensus guidelines for managing abnormal cervical cancer screening tests represent a major transition from algorithm-based guidelines to a risk-based approach. Patients are managed based on risk estimates derived from their current screening results and past history of normal or abnormal HPV test results, cytologic, and histologic findings. The guidelines use risk estimates derived from large screening studies and clinical action thresholds that are set by expert consensus to recommend that patients undergo treatment, colposcopy, follow-up at 1- or 3-year intervals, or return to routine screening at 5-year intervals. The new management guidelines are designed to streamline care, which can benefit patients during the pandemic. The guidelines focus on HPV testing as a mainstay of screening and management. Risk-based management recommendations can expedite diagnosis and treatment for high-risk patients, while decreasing unnecessary procedures for low-risk patients (Perkins et al., 2020).
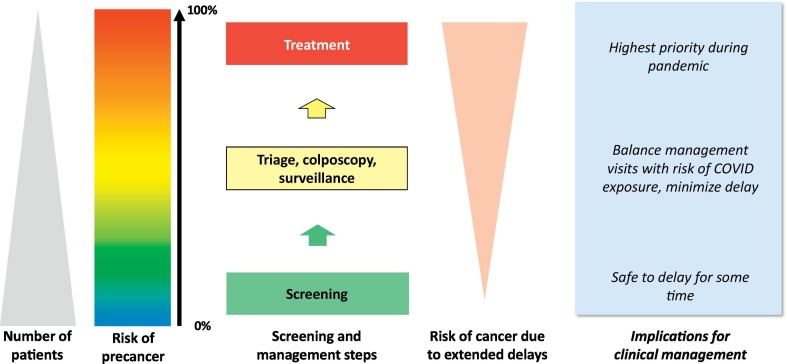
Risk-based guidelines can be used to determine which patients should be prioritized for care, which is critical to avoid delays in diagnosis among patients at highest risk for precancer and cancer, reduce the number of in-person visits among lower-risk patients to limit SARS-CoV-2 exposure, and to address the limited capacity for elective health services during the pandemic (Fig. 1 ). The 2019 risk-based management guidelines provide individualized, precise estimates of the likelihood of precancer (Egemen et al., 2020). Applying these guidelines streamlines care and minimizes in person visits while maximizing cancer prevention. Examples include setting a risk threshold for providing in person care, where high-risk patients in follow-up for abnormalities are seen, but low-risk patients participating in routine screening are deferred, or creating an ordered list of patients, from highest to lowest risk, to determine prioritization of in person visits. The risk threshold for inviting patients to in-person screening and management can be adapted based on the community risk of acquiring SARS-CoV-2 infections, which can rapidly change. Frequent change of address is an issue with some hard-to-reach populations. Some of the difficulties with obtaining adequate follow-up in these circumstances may be mitigated by community navigators (Nelson et al., 2020).
Fig. 1.
Principles of risk-based screening and management: Adapting clinical management during the pandemic.
5.3. Telehealth
Telehealth fills a crucial need for healthcare provision during the pandemic, and there may be important roles for telehealth in future clinical care (Monaghesh and Hajizadeh, 2020). Though telehealth has been in use for several years, laws requiring that patients and providers be in the same state or in specific clinical locations as well as lack of adequate reimbursement have inhibited adoption (Smith et al., 2020). During the pandemic, widespread restrictions of in-person services led to a rapid rise in telehealth. A survey conducted in May 2020 found that approximately 70% of patients were comfortable with phone or video visits, while 14% reported technology issues such as limited data plans, and 14% preferred in person visits (Primary Care Collaborative, 2020). Telehealth has been proposed as a means to improve rural patient healthcare access and care quality (Greenwood-Ericksen and Findley, 2020). However, a national survey of employer-based insurance noted a higher use of in-person and lower use of telemedicine in areas with lower income and higher proportions of Black and Hispanic residents (Eberly et al., 2020), indicating potential challenges to adoption in some communities.
Telehealth has particular relevance to cervical cancer screening. Self-collected HPV samples can facilitate continued screening during care disruptions and can also be used to accelerate catch-up screening as we move out of pandemic-related crisis care. Patients can be supported via telehealth to collect self-samples safely in their homes, and screening results can be discussed in subsequent virtual visits. This will reserve the limited in person visits for those needing colposcopy or treatment. Data examining cancer screening in federally qualified health center populations during the COVID pandemic found substantial disruptions to cervical, breast, and lung cancer screening, but fewer disruptions to colon cancer screening using at home fecal immunochemical testing (Fisher-Borne Prev Med). Continuation of legislation and reimbursement policies that facilitate telemedicine beyond the COVID pandemic are needed to expand telemedicine infrastructure and clinician training in best practices around delivery of these services (Rubin, 2020). Telehealth policies should include support of audio-only as well as video visits and ensure adequate broadband coverage to maintain access among certain groups including the elderly, low-income, Black and Hispanic populations (Hirko et al., 2020).
6. Conclusion
The COVID-19 pandemic has presented major challenges to societies world-wide, with health systems particularly affected. Evaluation and treatment of COVID patients combined with the need to reduce clinical services to comply with social distancing measures has led to major temporary reductions of elective health services. In many places, stresses on healthcare systems have further exposed inefficiencies, lack of healthcare access, and health disparities. Implementation of healthcare strategies is more challenging in the US system, which is decentralized and screens opportunistically, compared to organized screening programs. Important factors that could improve implementation include uniform guidelines that are supported by professional societies, and reimbursement that is tied to providing care according to guidelines.
The COVID-19 pandemic may not be our worst or only pandemic this century. It is important to learn from the current experience and build resilience into cervical cancer prevention systems to minimize the impacts of future disruptions in care (Steben et al., 2020). Knowledge of HPV natural history and carcinogenesis form the scientific underpinning of a functional, resilient system (Schiffman, 1994). One key component of this system will be ensuring access to screening with HPV testing for all eligible individuals. A negative screen using HPV testing is associated with a lower risk of developing precancer or cancer over the next 5–10 years compared to cytology alone and allows to safely delay screening in individuals with previously negative screening results for some time. Maximizing screening participation and ensuring follow-up of abnormal results are crucial to reducing cervical cancer rates overall as well as disparities related to income, location, and race/ethnicity (Castle et al., 2017; Musselwhite et al., 2016). HPV self-sampling obviates the need for an in person visit and can be safely continued during disruptions of in-person care (Cohen et al., 2020) and to facilitate catch-up of missed screenings. When screening results are abnormal, risk-based management guidelines are designed to expedite diagnosis and treatment for high-risk patients while reducing unnecessary procedures in low-risk patients (Perkins et al., 2020). When in-person care is limited, risk-based management can identify both high-risk patients who need in-person services and low-risk patients who can safely defer in-person care. Therefore, facilitating transition to newer, more efficient guidelines is crucial when developing prevention systems that are resilient to future disruptions in care.
Disclosures
The authors disclose no conflicts of interest.
Funding source
This work was supported by the Intramural Research Program of the National Cancer Institute.
Declaration of competing interest
The system does not accept the COI statement pdf in this category. It is uploaded as supplementary material. This is a placeholder.
References
- ACOG Committee Opinion No. 586 Health disparities in rural women. Obstet. Gynecol. 2014;123:384–388. doi: 10.1097/01.AOG.0000443278.06393.d6. [DOI] [PubMed] [Google Scholar]
- Adams C.H., Smith N.J., Wilbur D.C., et al. The relationship of obesity to the frequency of pelvic examinations: do physician and patient attitudes make a difference? Women Health. 1993;20:45–57. doi: 10.1300/J013v20n02_04. [DOI] [PubMed] [Google Scholar]
- Arbyn M., Smith S.B., Temin S., et al. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: updated meta-analyses. Bmj. 2018;363:k4823. doi: 10.1136/bmj.k4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldi E., Savastano S. 2020. Fear of Contagion: One of the Most Devious Enemies to Fight During the COVID-19 Pandemic. Disaster Medicine and Public Health Preparedness; pp. 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavis A.L., Gravitt P.E., Rositch A.F. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044–1050. doi: 10.1002/cncr.30507. [DOI] [PubMed] [Google Scholar]
- Benard V.B., Lawson H.W., Eheman C.R., et al. Adherence to guidelines for follow-up of low-grade cytologic abnormalities among medically underserved women. Obstet. Gynecol. 2005;105:1323–1328. doi: 10.1097/01.AOG.0000159549.56601.75. [DOI] [PubMed] [Google Scholar]
- Bhaskaran K., Douglas I., Forbes H., et al. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384:755–765. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Labor Statistics BoL. Bureau of Labor Statistics; 2021. The Employment Situation — December 2020. [Google Scholar]
- Burger E.A., Sy S., Kim J.J. 2020. Impact of COVID-19-Related Care Disruptions on Cervical Cancer Screening in the United States, Annual Society of Medical Decision Making Meeting (Virtual) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J.T., Ford C.L., Wallace S.P., et al. Intersection of living in a rural versus urban area and race/ethnicity in explaining access to health care in the United States. Am. J. Public Health. 2016;106:1463–1469. doi: 10.2105/AJPH.2016.303212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle E.E., Rodriguez C., Walker-Thurmond K., et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- Castle P.E., Kinney W.K., Cheung L.C., et al. Why does cervical cancer occur in a state-of-the-art screening program? Gynecol. Oncol. 2017;146:546–553. doi: 10.1016/j.ygyno.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle P.E., Kinney W.K., Xue X., et al. Effect of several negative rounds of human papillomavirus and cytology co-testing on safety against cervical cancer: an observational cohort study. Ann. Intern. Med. 2018;168:20–29. doi: 10.7326/M17-1609. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; 2020. Infection Control Guidance for Healthcare Professionals about Coronavirus (COVID-19) [Google Scholar]
- Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; 2020. TeenVaxView.https://www.cdc.gov/vaccines/imz-managers/coverage/teenvaxview/index.html Centers for Disease Control. [Google Scholar]
- Chang J., Wignadasan W., Kontoghiorghe C., et al. Restarting elective orthopaedic services during the COVID-19 pandemic: do patients want to have surgery? Bone Joint Open. 2020;1:267–271. doi: 10.1302/2633-1462.16.BJO-2020-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M.A., Fetterman B., Cheung L.C., et al. Epidemiologic evidence that excess body weight increases risk of cervical cancer by decreased detection of precancer. J. Clin. Oncol. 2018;36:1184–1191. doi: 10.1200/JCO.2017.75.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M.A., Devesa S.S., Harvey S.V., et al. Hysterectomy-corrected uterine corpus cancer incidence trends and differences in relative survival reveal racial disparities and rising rates of nonendometrioid cancers. J. Clin. Oncol. 2019;37:1895–1908. doi: 10.1200/JCO.19.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M.A., Massad L.S., Khan M.J., et al. Challenges associated with cervical cancer screening and management in obese women: a provider perspective. J. Low Genit. Tract. Dis. 2020;24:184–191. doi: 10.1097/LGT.0000000000000506. [DOI] [PubMed] [Google Scholar]
- Cohen M.A., Powell A.M., Coleman J.S., et al. Special ambulatory gynecologic considerations in the era of coronavirus disease 2019 (COVID-19) and implications for future practice. Am. J. Obstet. Gynecol. 2020;223:372–378. doi: 10.1016/j.ajog.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker A.L., Eggleston K.S., Du X.L., et al. Ethnic disparities in cervical cancer survival among Medicare eligible women in a multiethnic population. Int. J. Gynecol. Cancer. 2009;19:13–20. doi: 10.1111/IGC.0b013e318197f343. [DOI] [PubMed] [Google Scholar]
- Collins Y., Holcomb K., Chapman-Davis E., et al. Gynecologic cancer disparities: a report from the health disparities taskforce of the Society of Gynecologic Oncology. Gynecol. Oncol. 2014;133:353–361. doi: 10.1016/j.ygyno.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry S.J., Krist A.H., Owens D.K., et al. Screening for cervical cancer: US preventive services task force recommendation statement. Jama. 2018;320:674–686. doi: 10.1001/jama.2018.10897. [DOI] [PubMed] [Google Scholar]
- Datta G.D., Colditz G.A., Kawachi I., et al. Individual-, neighborhood-, and state-level socioeconomic predictors of cervical carcinoma screening among U.S. black women: a multilevel analysis. Cancer. 2006;106:664–669. doi: 10.1002/cncr.21660. [DOI] [PubMed] [Google Scholar]
- Doubeni C.A., Gabler N.B., Wheeler C.M., et al. Timely follow-up of positive cancer screening results: a systematic review and recommendations from the PROSPR consortium. CA Cancer J. Clin. 2018;68:199–216. doi: 10.3322/caac.21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs L.S., Smith J.S., Scarinci I., et al. The disparity of cervical cancer in diverse populations. Gynecol. Oncol. 2008;109:S22–S30. doi: 10.1016/j.ygyno.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Eberly L.A., Kallan M.J., Julien H.M., et al. Patient characteristics associated with telemedicine access for primary and specialty ambulatory care during the COVID-19 pandemic. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egemen D., Cheung L.C., Chen X., et al. Risk estimates supporting the 2019 ASCCP risk-based management consensus guidelines. J. Low Genit. Tract. Dis. 2020;24:132–143. doi: 10.1097/LGT.0000000000000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleston K.S., Coker A.L., Williams M., et al. Cervical cancer survival by socioeconomic status, race/ethnicity, and place of residence in Texas, 1995-2001. J. Women’s Health (Larchmt) 2006;15:941–951. doi: 10.1089/jwh.2006.15.941. [DOI] [PubMed] [Google Scholar]
- Eggleston K.S., Coker A.L., Luchok K.J., et al. Adherence to recommendations for follow-up to abnormal pap tests. Obstet. Gynecol. 2007;109:1332–1341. doi: 10.1097/01.AOG.0000266396.25244.68. [DOI] [PubMed] [Google Scholar]
- Espey D., Paisano R., Cobb N. Regional patterns and trends in cancer mortality among American Indians and Alaska natives, 1990-2001. Cancer. 2005;103:1045–1053. doi: 10.1002/cncr.20876. [DOI] [PubMed] [Google Scholar]
- Fernandez M.E., Savas L.S., Lipizzi E., et al. Cervical cancer control for Hispanic women in Texas: strategies from research and practice. Gynecol. Oncol. 2014;132(Suppl. 1):S26–S32. doi: 10.1016/j.ygyno.2013.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontham E.T.H., Wolf A.M.D., Church T.R., et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J. Clin. 2020;70:321–346. doi: 10.3322/caac.21628. [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration . Food and Drug Administration; 2019. FDA Executive Summary: New Approaches in the Evaluation for High-Risk Human Papillomavirus Nucleic Acid Detection Devices. [Google Scholar]
- Franchini S., Spessot M., Landoni G., et al. Stranger months: how SARS-CoV-2, fear of contagion, and lockdown measures impacted attendance and clinical activity during February and March 2020 at an Urban Emergency Department in Milan. Disaster Med. Public Health Preparedness. 2020:1–10. doi: 10.1017/dmp.2020.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman H.P.W.B. 2005. Excess Cervical Cancer Mortality: A Marker for Low Access to Health Care in Poor Communities, in National Cancer Institute CtRCHD (ed). Rockville, MD. [Google Scholar]
- Gage J.C., Schiffman M., Katki H.A., et al. Reassurance against future risk of precancer and cancer conferred by a negative human papillomavirus test. J. Natl. Cancer Inst. 2014;106 doi: 10.1093/jnci/dju153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland F.D., Hunt W.C., Key C.R. Trends in the survival of American Indian, Hispanic, and non-Hispanic white cancer patients in New Mexico and Arizona, 1969-1994. Cancer. 1998;82:1769–1783. doi: 10.1002/(sici)1097-0142(19980501)82:9<1784::aid-cncr26>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Greenwood-Ericksen M.B.D.A.S., Findley S. 2020. Transforming the Rural Health Care Paradigm. JAMA Health Forum. [DOI] [PubMed] [Google Scholar]
- Hart C. 2020. The Effect of COVID-19 on Immunization Rates. [Google Scholar]
- Henley S.J., Anderson R.N., Thomas C.C., et al. Invasive cancer incidence, 2004-2013, and deaths, 2006-2015, in nonmetropolitan and metropolitan counties - United States. MMWR Surveill. Summ. 2017;66:1–13. doi: 10.15585/mmwr.ss6614a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirko K.A., Kerver J.M., Ford S., et al. Telehealth in response to the COVID-19 pandemic: implications for rural health disparities. J. Am. Med. Inform. Assoc. 2020;27:1816–1818. doi: 10.1093/jamia/ocaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner-Johnson W., Dobbertin K., Iezzoni L.I. Disparities in receipt of breast and cervical cancer screening for rural women age 18 to 64 with disabilities. Womens Health Issues. 2015;25:246–253. doi: 10.1016/j.whi.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Housing Assistance Council . Housing Assistance Council; 2020. Rural Unemployment Rate Declines, but 1.8 Million Rural Workers Still Unemployed.http://www.ruralhome.org/whats-new/mn-coronavirus/1864-rural-unemployment-rate-declines-but-18-million-rural-workers-still-unemployed#:~:text=The%20most%20recent%20data%20from,the%20COVID%2D19%20health%20crisis [Google Scholar]
- Hung P., Henning-Smith C.E., Casey M.M., et al. Access to obstetric services in rural counties still declining, with 9 percent losing services, 2004–14. Health Affairs (Project Hope) 2017;36:1663–1671. doi: 10.1377/hlthaff.2017.0338. [DOI] [PubMed] [Google Scholar]
- Islami F., Fedewa S.A., Jemal A. Trends in cervical cancer incidence rates by age, race/ethnicity, histological subtype, and stage at diagnosis in the United States. Prev. Med. 2019;123:316–323. doi: 10.1016/j.ypmed.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Kim J.J., Burger E.A., Regan C., et al. Agency for Healthcare Research and Quality (US); Rockville (MD): 2018. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews, Screening for Cervical Cancer in Primary Care: A Decision Analysis for the U.S. Preventive Services Task Force. [PubMed] [Google Scholar]
- Ku L., Brantley E. Widening social and health inequalities during the COVID-19 pandemic. JAMA Health Forum. 2020;1 doi: 10.1001/jamahealthforum.2020.0721. e200721-e200721. [DOI] [PubMed] [Google Scholar]
- Lei J., Ploner A., Elfström K.M., et al. HPV vaccination and the risk of invasive cervical cancer. N. Engl. J. Med. 2020;383:1340–1348. doi: 10.1056/NEJMoa1917338. [DOI] [PubMed] [Google Scholar]
- Lulu Yu S.A.S., Mary C. White: rural–urban and racial/ethnic disparities in invasive cervical cancer incidence in the United States, 2010–2014. Prev. Chronic Dis. 2019;16 doi: 10.5888/pcd16.180447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaughlin K.L., Jacobson R.M., Radecki Breitkopf C., et al. Trends over time in pap and pap-HPV cotesting for cervical cancer screening. J. Women’s Health. 2019;28:244–249. doi: 10.1089/jwh.2018.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid U., Kandasamy S., Farrah K., et al. Women’s preferences and experiences of cervical cancer screening in rural and remote areas: a systematic review and qualitative meta-synthesis. Rural Remote Health. 2019;19:5190. doi: 10.22605/RRH5190. [DOI] [PubMed] [Google Scholar]
- Maruthur N.M., Bolen S.D., Brancati F.L., et al. The association of obesity and cervical cancer screening: a systematic review and meta-analysis. Obesity (Silver Spring) 2009;17:375–381. doi: 10.1038/oby.2008.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast C., Munoz del Rio A. 2020. Delayed Cancer Screenings—A Second Look. [Google Scholar]
- Meites E., Szilagyi P.G., Chesson H.W., et al. Human papillomavirus vaccination for adults: updated recommendations of the advisory committee on immunization practices. MMWR Morb. Mortal. Wkly Rep. 2019;68:698–702. doi: 10.15585/mmwr.mm6832a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikow J., Henderson J.T., Burda B.U., et al. Screening for cervical cancer with high-risk human papillomavirus testing: updated evidence report and systematic review for the US preventive services task force. Jama. 2018;320:687–705. doi: 10.1001/jama.2018.10400. [DOI] [PubMed] [Google Scholar]
- Melvin S.C., Wiggins C., Burse N., et al. The role of public health in COVID-19 emergency response efforts from a rural health perspective. Prev. Chronic Dis. 2020;17 doi: 10.5888/pcd17.200256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B.A., Chu K.C., Hankey B.F., et al. Cancer incidence and mortality patterns among specific Asian and Pacific islander populations in the U.S. Cancer Causes Control. 2008;19:227–256. doi: 10.1007/s10552-007-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.J., Xu L., Qin J., et al. Impact of COVID-19 on cervical cancer screening rates among women aged 21-65 years in a large integrated health care system - Southern California, January 1-September 30, 2019, and January 1-September 30, 2020. MMWR Morb. Mortal. Wkly Rep. 2021;70:109–113. doi: 10.15585/mmwr.mm7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghesh E., Hajizadeh A. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health. 2020;20:1193. doi: 10.1186/s12889-020-09301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.T., Ricaldi J.N., Rose C.E., et al. Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5–18, 2020–22 states, February–June 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:1122–1126. doi: 10.15585/mmwr.mm6933e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland A., Herlihy C., Tynan M.A., et al. Timing of state and territorial COVID-19 stay-at-home orders and changes in population movement - United States, march 1-may 31, 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:1198–1203. doi: 10.15585/mmwr.mm6935a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselwhite L.W., Oliveira C.M., Kwaramba T., et al. Racial/ethnic disparities in cervical cancer screening and outcomes. Acta Cytol. 2016;60:518–526. doi: 10.1159/000452240. [DOI] [PubMed] [Google Scholar]
- Nelson H.D., Cantor A., Wagner J., et al. Effectiveness of patient navigation to increase cancer screening in populations adversely affected by health disparities: a meta-analysis. J. Gen. Intern. Med. 2020;35:3026–3035. doi: 10.1007/s11606-020-06020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan J., Renderos T.B., Hynson J., et al. Barriers to cervical cancer screening and follow-up care among black women in Massachusetts. J. Obstet. Gynecol. Neonatal. Nurs. 2014;43:580–588. doi: 10.1111/1552-6909.12488. [DOI] [PubMed] [Google Scholar]
- Oliver S.E., Unger E.R., Lewis R., et al. Prevalence of human papillomavirus among females after vaccine introduction-National Health and nutrition examination survey, United States, 2003-2014. J. Infect. Dis. 2017;216:594–603. doi: 10.1093/infdis/jix244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver K., McCorkell C., Pister I., et al. Improving HPV vaccine delivery at school-based health centers. Hum. Vaccin Immunother. 2019;15:1870–1877. doi: 10.1080/21645515.2019.1578596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins R.B., Guido R.S., Castle P.E., et al. 2019 ASCCP risk-based management consensus guidelines for abnormal cervical cancer screening tests and cancer precursors. J. Low Genit. Tract. Dis. 2020;24:102–131. doi: 10.1097/LGT.0000000000000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman N.J., Snijders P.J.F., Kenter G.G., et al. HPV-based cervical screening: rationale, expectations and future perspectives of the new Dutch screening programme. Prev. Med. 2019;119:108–117. doi: 10.1016/j.ypmed.2018.12.021. [DOI] [PubMed] [Google Scholar]
- President’s Cancer Panel . President’s Cancer Panel; 2020. Improving Resilience and Equity in Cervical Cancer Screening: Lessons from COVID-19 and Beyond.https://prescancerpanel.cancer.gov/2020/improving-resilience-and-equity-cervical-cancer-screening-lessons-covid-19-and-beyond [Google Scholar]
- Primary Care Collaborative . Primary Care Collaborative; 2020. Primary Care & COVID-19: Round 23 Survey.https://www.pcpcc.org/2020/11/23/primary-care-covid-19-round-23-survey [Google Scholar]
- Probst J.C.M.C., Glover S.H., Samuels M.E. Person and place: the compounding effects of race/ethnicity and rurality on health. Am. J. Public Health. 2004;94:1695–1703. doi: 10.2105/ajph.94.10.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. COVID-19’s crushing effects on medical practices, some of which might not survive. JAMA. 2020;324:321–323. doi: 10.1001/jama.2020.11254. [DOI] [PubMed] [Google Scholar]
- Rutter C.M., Kim J.J., Meester R.G.S., et al. Effect of time to diagnostic testing for breast, cervical, and colorectal cancer screening abnormalities on screening efficacy: a modeling study. Cancer Epidemiol. Biomark. Prev. 2018;27:158–164. doi: 10.1158/1055-9965.EPI-17-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saslow D., Solomon D., Lawson H.W., et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J. Clin. 2012;62:147–172. doi: 10.3322/caac.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saville M., Hawkes D., McLachlan E., et al. Self-collection for under-screened women in a National Cervical Screening Program: pilot study. Curr. Oncol. 2018;25:e27–e32. doi: 10.3747/co.25.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman M.H. Epidemiology of cervical human papillomavirus infections. Curr. Top. Microbiol. Immunol. 1994;186:55–81. doi: 10.1007/978-3-642-78487-3_4. [DOI] [PubMed] [Google Scholar]
- Schiffman M., Doorbar J., Wentzensen N., et al. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Primers. 2016;2:16086. doi: 10.1038/nrdp.2016.86. [DOI] [PubMed] [Google Scholar]
- Semrad T.J., Tancredi D.J., Baldwin L.M., et al. Geographic variation of racial/ethnic disparities in colorectal cancer testing among medicare enrollees. Cancer. 2011;117:1755–1763. doi: 10.1002/cncr.25668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavers V.L., Brown M.L. Racial and ethnic disparities in the receipt of cancer treatment. J. Natl. Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- Singh G.K. Rural-urban trends and patterns in cervical cancer mortality, incidence, stage, and survival in the United States, 1950-2008. J. Community Health. 2012;37:217–223. doi: 10.1007/s10900-011-9439-6. [DOI] [PubMed] [Google Scholar]
- Singh G.K., Jemal A. Socioeconomic and racial/ethnic disparities in Cancer mortality, incidence, and survival in the United States, 1950–2014: over six decades of changing patterns and widening inequalities. J. Environ. Public Health. 2017;2017:1–19. doi: 10.1155/2017/2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.C., Thomas E., Snoswell C.L., et al. Telehealth for global emergencies: implications for coronavirus disease 2019 (COVID-19) J. Telemed. Telecare. 2020;26:309–313. doi: 10.1177/1357633X20916567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z., Giurato M., Lillehaugen T., et al. 2020. Economic and Clinical Impact of Covid-19 on Provider Practices in Massachusetts. September 11, 2020. [Google Scholar]
- Spencer J.C., Calo W.A., Brewer N.T. Disparities and reverse disparities in HPV vaccination: a systematic review and meta-analysis. Prev. Med. 2019;123:197–203. doi: 10.1016/j.ypmed.2019.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steben M., Norris T., Rosberger Z. COVID-19 Won’t be the last (or worst) pandemic: It’s time to build resilience into our cervical Cancer elimination goals. J. Obstet. Gynaecol. Can. 2020;42:1195–1196. doi: 10.1016/j.jogc.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcuoglu O., Yazici O., Ozet A., et al. 2020. Harmful Consequences of COVID-19 Fear in Patients with Cancer. BMJ Supportive & Palliative Care. [DOI] [PubMed] [Google Scholar]
- The Physicians Foundation . The Physicians Foundation; 2020. 2020 Survey of America’s Physicians COVID-19 Impact Edition.https://physiciansfoundation.org/wp-content/uploads/2020/12/2020-Survey-of-Americas-Physicians_Exec-Summary.pdf [Google Scholar]
- Tribble S.J. Kaiser Health News; 2020. Prognosis for Rural Hospitals Worsens with Pandemic. [Google Scholar]
- U.S. Centers for Medicare & Medicaid Services. U.S. Centers for Medicare & Medicaid Services.; 2021. Medicare Coverage.https://www.medicare.gov/coverage [Google Scholar]
- U.S. Department of Health and Human Services . U.S. Department of Health and Human Services; 2020. Cancer and American Indians/Alaska Natives, U.S. Department of Health and Human Services Office of Minority Health.https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=31 [Google Scholar]
- Walker T.Y., Elam-Evans L.D., Yankey D., et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years - United States, 2018. MMWR Morb. Mortal. Wkly Rep. 2019;68:718–723. doi: 10.15585/mmwr.mm6833a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.W., Prieto J., Ikeda D.S., et al. Perforated appendicitis: an unintended consequence during the Coronavirus-19 pandemic. Mil. Med. 2020 doi: 10.1093/milmed/usaa527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts L., Joseph N., Velazquez A., et al. Understanding barriers to cervical cancer screening among Hispanic women. Am. J. Obstet. Gynecol. 2009;201(199):e1–e8. doi: 10.1016/j.ajog.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Wee C.C., McCarthy E.P., Davis R.B., et al. Screening for cervical and breast cancer: is obesity an unrecognized barrier to preventive care? Ann. Intern. Med. 2000;132:697–704. doi: 10.7326/0003-4819-132-9-200005020-00003. [DOI] [PubMed] [Google Scholar]
- Wentzensen N., Clarke M.A. Cancer Epidemiology Biomarkers & Prevention in press; 2021. Cervical Cancer Screening - Past, Present, and Future. [DOI] [PubMed] [Google Scholar]
- Wentzensen N., Walker J.L., Gold M.A., et al. Multiple biopsies and detection of cervical cancer precursors at colposcopy. J. Clin. Oncol. 2015;33:83–89. doi: 10.1200/JCO.2014.55.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzensen N., Schiffman M., Palmer T., et al. Triage of HPV positive women in cervical cancer screening. J. Clin. Virol. 2016;76(Suppl. 1):S49–s55. doi: 10.1016/j.jcv.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzensen N., Massad L.S., Mayeaux E.J., Jr., et al. Evidence-based consensus recommendations for colposcopy practice for cervical Cancer prevention in the United States. J. Low Genit. Tract. Dis. 2017;21:216–222. doi: 10.1097/LGT.0000000000000322. [DOI] [PubMed] [Google Scholar]
- Wilson R.J., Ryerson A.B., Singh S.D., et al. Cancer incidence in Appalachia, 2004-2011. Cancer Epidemiol. Biomark. Prev. 2016;25:250–258. doi: 10.1158/1055-9965.EPI-15-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo W., Kim S., Huh W.K., et al. Recent trends in racial and regional disparities in cervical cancer incidence and mortality in United States. PLoS One. 2017;12 doi: 10.1371/journal.pone.0172548. [DOI] [PMC free article] [PubMed] [Google Scholar]