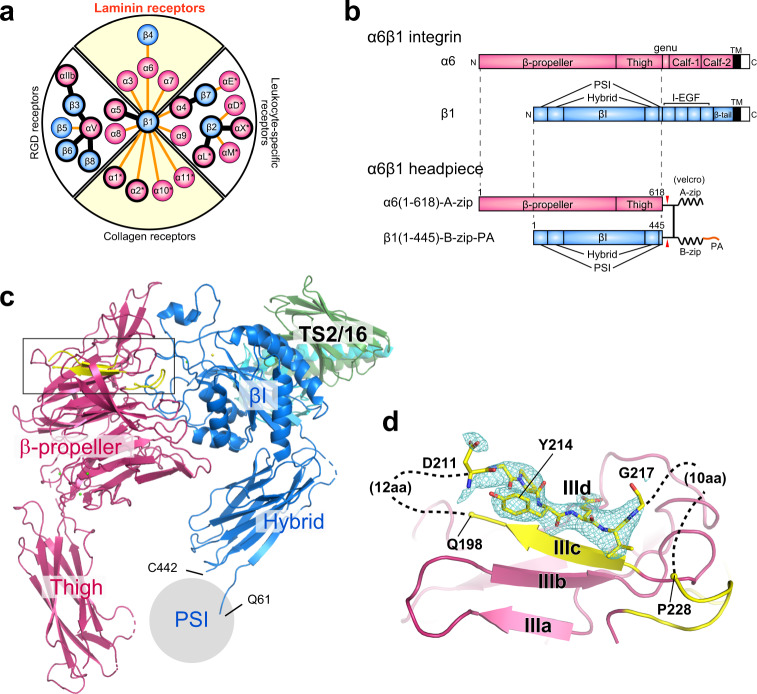
Fig. 1. Crystal structure of the α6β1 integrin headpiece in complex with TS2/16 Fv-clasp.
a Integrin α/β pairs and classification. 18 α subunits (red circles, with the αI domain-containing ones indicated by asterisks) and 8 β subunits (blue circles) found in human forming 24 heterodimeric pairs are represented by the α-β connecting lines. Integrin subunits or heterodimers with at least partial ectodomain structures determined are denoted by thick circles. Each heterodimer falls into one of the four distinct classes. Modified from the Fig. 1 of ref. 1. b Domain organization of α6β1 integrin and design of the α6β1 headpiece construct used for the structural analysis. c Ribbon presentation of the overall structure. Integrin α6 and β1 subunits are colored in hot pink and sky blue, respectively, and TS2/16 VH-SARAH and TS2/16 VL-SARAH composing TS2/16 Fv-clasp are in green and cyan, respectively. The location of the PSI domain (not included in the model) is denoted by gray circle, with the boundary residues (Q61 and C442) labeled. d An expanded view of the blade III β-sheet of the α6 subunit-β-propeller. The region indicated by a rectangle in (c) containing the X1 region (yellow) is shown with an Fo-Fc electron density map corresponding to the outermost strand (IIId) contoured at 3.0 σ. The modeled heptapeptide 211DGPYEVG217 fitted in the map is also shown as stick models.