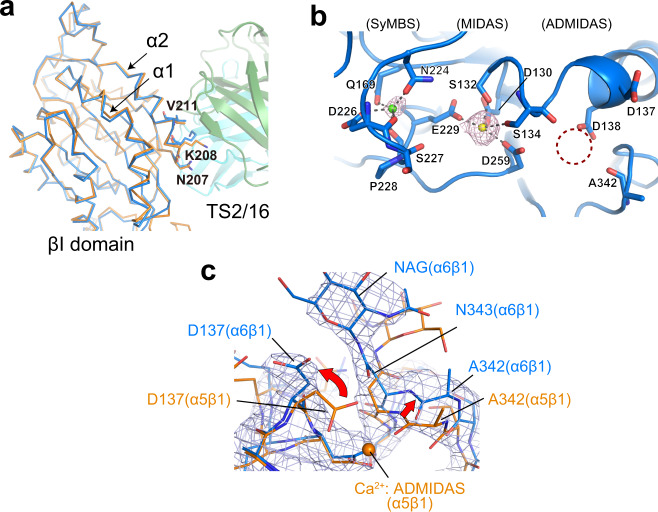
Fig. 2. Structure of the βI domain.
a Structural comparison of β1 integrins before and after the TS2/16 binding. The β1 subunit portions from the TS2/16-bound α6β1 headpiece (sky blue) and the unbound α5β1 headpiece (PDB ID:4wjk, orange) are superposed at the βI domain and shown as Cα-tracing. TS2/16 Fv-clasp bound to α6β1 is shown as a translucent ribbon diagram. The α2 helix of βI domain harboring TS2/16 epitope residues (N207/K208/V211) and the adjacent α1 helix are labeled. b Metal ion coordination sites in the α6β1 headpiece structure. Simulated-annealing Fo-Fc omit map for metal ions contoured at 2.5 σ is shown in magenta. Ca2+ and Mg2+ ions assigned in the SyMBS and the MIDAS are shown as green and yellow spheres, respectively. No electron density was observed at the ADMIDAS (dotted circle). c 2Fo-Fc electron density map contoured at 1.2 σ around the ADMIDAS region in the crystal structure of the α6β1-TS2/16 Fv-clasp complex (sky blue), superposed onto the α5β1 headpiece structure (orange) as in a. Ca2+ ion bound to the α5β1 headpiece is shown as an orange sphere.