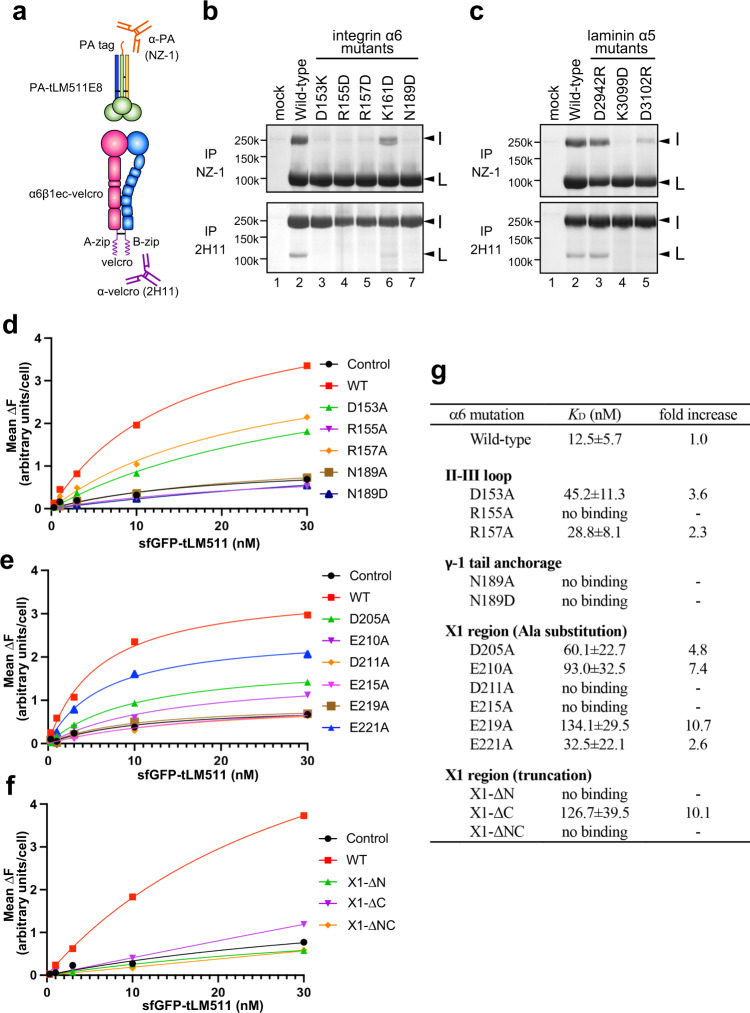
Fig. 6. Mutagenesis of the interface residues.
a Schematics of the α6β1 integrin ectodomain (α6β1ec-velcro) and tLM511 (PA-tLM511) constructs used for the pull-down binding assay. Binding of integrin mutants to wild-type laminin b and laminin mutants to wild-type integrin c. Each construct was transfected into Expi293F cells, and culture media of each pair of integrin/laminin were mixed and subjected to immunoprecipitation using anti-PA tag antibody (NZ-1, upper panel) or anti-velcro tag antibody (2H11, lower panel), followed by SDS-PAGE analysis under non-reducing condition. Band positions of α6β1ec-velcro and PA-tLM511 are indicated by I and L, respectively. Immunoprecipitation experiments were repeated twice. Binding analysis of sfGFP-fused tLM511 to the integrin mutants expressed on the cell surface. Expi293F cells transiently expressing α6β1 integrins with the II-III loop mutations d, X1-region mutations e, and loop truncation mutations f were incubated with increasing concentrations of sfGFP-tLM511 to measure its binding as described in the “Method” section. Each panel shows data from a representative experiment out of three independent ones. g Laminin binding affinity of mutant α6β1 integrins. Equilibrium dissociation constants (KD) derived from the binding isotherms like the ones shown in d–f by non-linear regression analysis are shown. When the binding was below the level of control non-transfected cells (black circles in d–f), that mutant integrin is judged as no binding. Data are mean ± SD of three independent experiments. Relative impact of each mutation on the binding is expressed as the fold-increase in the KD value from the wild-type. Uncropped gel images and source data are provided in the Source Data file.