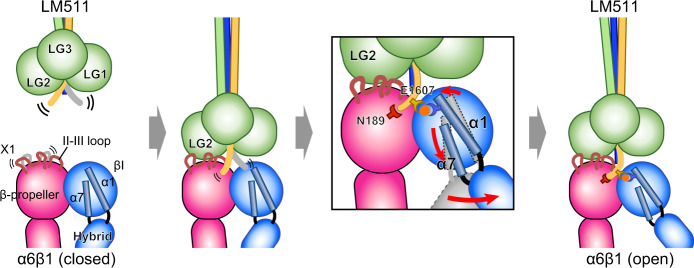
Fig. 7. Hypothetical model of the LM511 recognition by α6β1 integrin.
In the first step, a long-range electrostatic search via the mobile and charged X1 region attracts the LG domain of laminin to integrin (the first panel). The II-III loop fixes the relative orientation between the integrin and laminin (the second panel). The flexible γ1-tail is fixed via two anchor points (i.e., LMγ1 P1609-α6 N189 and LMγ1 E1607-MIDAS), which induces local conformational rearrangement of α1 and α7 helices in the integrin βI domain, making the hybrid domain swing out (the third panel). Eventually, α6β1 is converted into the extended-open conformation (the last panel).