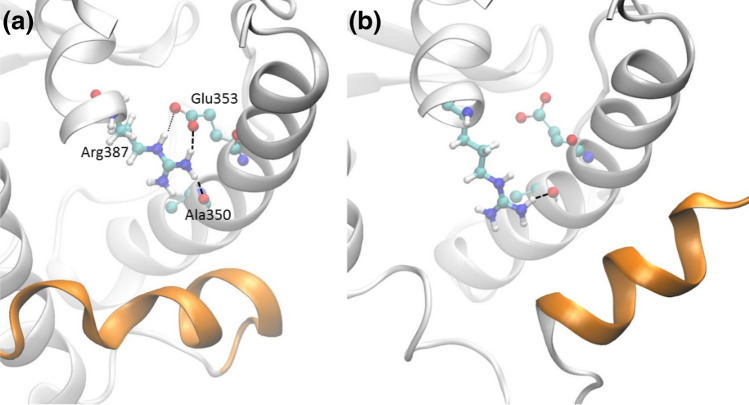
Figure 3.
Formation of hydrogen bonds between the mutant residue 387 with other residues within the helix 3 (H3) at the active (a) and inactive (b) states. At the active state, Arg387 forms one stable hydrogen bond (bold dashed lines) with residues Ala350 and Glu353 each, and an additional one (light dotted line) with Glu353, which appears in ~ 65% of the entire simulations. At the inactive state, the side chain of Arg387 only forms one stable hydrogen bond with Ala350 (side chain of Glu353 forms hydrogen bonds with Arg394 instead, with 126% frequency of occurrence; the frequency is 15% in the active state). The helices H4, H8 and H9 are removed from both figures for reasons of clarity.