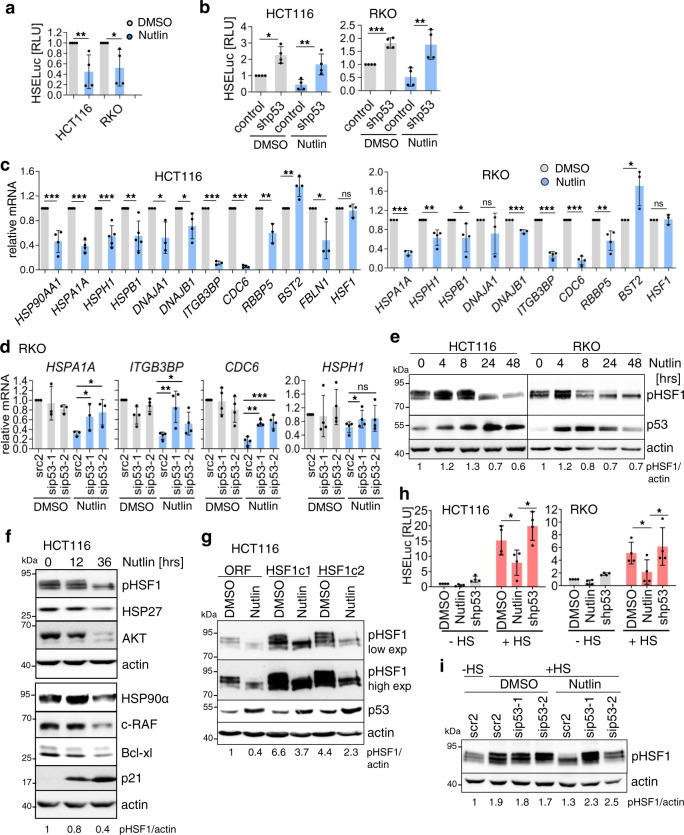
Fig. 3. Activated WTp53 represses HSF1 activity in human colorectal cancer cells.
a Luciferase reporter assay for heat-shock response elements (HSE) in HCT116 and RKO cells treated with 10 µM Nutlin/DMSO for 24 h. b HSE luciferase assay as in a after depletion of WTp53 by shRNA. Control, scramble shRNA. Forty-eight hours post transfection, cells were treated with Nutlin/DMSO (10 µM) for 24 h. c Chaperone-dependent and -independent HSF1 target gene expression in indicated cells treated with 10 µM Nutlin/DMSO for 24 h. qRT-PCR normalized to RPLP0 or HPRT1 mRNA. d HSF1 target gene expression in RKO cells upon WTp53 depletion. Forty-eight hours post transfection with sip53RNAs or scrambled control siRNA, cells were treated with Nutlin/DMSO (10 µM) for 24 h. qRT-PCR as in c. e Activated WTp53 is correlated with suppression of pSer326-HSF1, the key marker of HSF1 activity. Indicated cells were treated ±10 µM Nutlin. f Repression of HSF1 targets and destabilization of Hsp90 clients after p53 activation. Cells were treated with 10 µM Nutlin/DMSO. g Stably HSF1-overexpressing HCT116 subclones (HSF1c1 and HSF1c2) or empty vector clone (ORF) were treated with Nutlin/DMSO for 24 h. pSer326-HSF1 shown with short and long exposures. h Nutlin represses HSF1 activity in heat-shocked cells, rescued by p53 knockdown. HSE luciferase assay as in b. Cells were treated with 10 µM Nutlin/DMSO for 24 h. During the final 2 h, cells were heat-shocked for 1 h at 42 °C followed by 1 h recovery. i The heat-shock response is attenuated by Nutlin, rescued by p53 knockdown. Cells were transfected with sip53RNAs or scrambled RNA (scr2) and treated as in h. Immunoblot for pSer326-HSF1. e–g, i Immunoblots. Actin, loading control. pHSF1/actin, pHSF1 densitometry normalized to loading control. Source data are provided as Source data file. a–d, h Student’s t test, two-sided. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001; ns not significant. a, b, h Mean ± SD of ≥3 independent experiments, each measured in triplicates. c, d Mean ± SD of ≥2 independent experiments, at least one with a technical replicate. Relative values given in [ratio (2−ddCT)].