Abstract
Purpose
The aim of the study was to report clinical features, contributing factors and outcome of patients with coronavirus disease 2019 (COVID‐19)‐associated mucormycosis (CAM).
Methods
A cross‐sectional descriptive multicentre study was conducted on patients with biopsy‐proven mucormycosis with RT‐PCR‐confirmed COVID‐19 from April to September 2020. Demographics, the time interval between COVID‐19 and mucormycosis, underlying systemic diseases, clinical features, course of disease and outcomes were collected and analysed.
Results
Fifteen patients with COVID‐19 and rhino‐orbital mucormycosis were observed. The median age of patients was 52 years (range 14–71), and 66% were male. The median interval time between COVID‐19 disease and diagnosis of mucormycosis was seven (range: 1–37) days. Among all, 13 patients (86%) had diabetes mellitus, while 7 (46.6%) previously received intravenous corticosteroid therapy. Five patients (33%) underwent orbital exenteration, while seven (47%) patients died from mucormycosis. Six patients (40%) received combined antifungal therapy and none that received combined antifungal therapy died.
Conclusion
Clinicians should be aware that mucormycosis may be complication of COVID‐19 in high‐risk patients. Poor control of diabetes mellitus is an important predisposing factor for CAM. Systematic surveillance for control of diabetes mellitus and educating physician about the early diagnosis of CAM are suggested.
Keywords: COVID‐19, diabetes, mucormycosis, Orbital mucormycosis, rhino‐orbital infection, SARS‐CoV‐2 co‐infection
1. BACKGROUND
Coronavirus disease 2019 (COVID‐19) is devastatingly sweeping throughout the world and became the pandemic threat. 1 Although the majority of the COVID‐19 cases will experience mild to moderate form of respiratory illness and improved without taking special medications, aged individuals and those with underlying medical conditions are more probably to develop the severe form of COVID‐19. 2 , 3 , 4 , 5 The infection in these patients progresses rapidly evolving respiratory deterioration and may lead to acute respiratory distress syndrome (ARDS). 4 , 5 , 6 The bacterial and fungal co‐infections have been documented in patients suffering from severe acute respiratory syndrome (SARS), Middle East respiratory syndrome and influenza, but the knowledge on co‐infections particularly fungal infections among critically ill COVID‐19 patients is limited. 7 Accordingly, paying attention to opportunistic fungal infections in COVID‐19 patients, 6 , 8 , 9 with a list of predisposing factors, is important for healthcare providers who are confronting the COVID‐19 pandemic. 1 , 3 , 10 COVID‐19 patients suffering from ARDS, those who require a long stay in an intensive care unit (ICU) and mechanical ventilation, taking high doses of corticosteroids, immunomodulators, interleukin antagonists and broad‐spectrum antibiotics, are at manifold risk to develop fungal infections such as mucosal candidiasis, aspergillosis, mucormycosis, pneumocystis jiroveci pneumonia and candidemia. 1 , 3 , 6 , 10 , 11 , 12 , 13 , 14 There is a paucity of data regarding the rate of COVID‐19‐associated mucormycosis (CAM). 15 To the best of our knowledge, the rate, clinical features and course of CAM in patients who simultaneously infected with COVID‐19 has never reported before. We aimed to investigate the clinical features, temporal relationship to COVID‐19 and course of patients with CAM.
2. METHODOLOGY
2.1. Study design
A cross‐sectional descriptive study on biopsy‐proven mucormycosis patients with laboratory‐confirmed COVID‐19 was conducted with collaboration of five COVID‐19 hospitalised canters in Tehran, Iran (Imam Khomeini hospital complex, Farabi hospital, Imam Hossein hospital, Shariati hospital and Firoozgar hospital) from April to September 2020. The protocol of this study was in accordance with the principles established by the Declaration of Helsinki and approved by the ethics committee of Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.VCR.REC.1399.152).
2.2. Case definition, data collection and histopathological examination
Patients with following criteria included the following: 1. Angio‐invasive mucormycosis should be confirmed on histopathologic examination using haematoxylin and eosin (H&E) staining 2. A verified case of COVID‐19 defined as documentation of a positive result of real‐time reverse transcriptase polymerase chain reaction (RT‐PCR) for nasopharyngeal or oropharyngeal swab, tracheal aspirate and/or bronchoalveolar lavage (BAL) samples 3. The interval between two infections should not be more than 3 months. Clinical and para‐clinical data including demographics, underlying diseases, clinical features and outcome were collected. The COVID‐19 infection was categorised according to World Health Organization (WHO) guideline: mild, moderate and severe. 16 For attributing the clinical form of mucormycosis, the location and extension of the disease, computerised tomography (CT) scan of the orbit, paranasal sinuses and lung were used for all patients as the initial imaging study. Gadolinium‐enhanced magnetic resonance imaging (MRI) of the orbits, brain and paranasal sinuses was also performed for patients who needed based on their symptoms. Clinical characteristics of each patient who met inclusion criteria were recorded. Patients were informed, and written consent was obtained after explaining that their clinical and biological data may be used for research purposes. Clinical radiological investigations, operative and outpatient follow‐up data were recorded and analysed for possible predisposing factors, demographic profile, clinical features of COVID‐19 and mucormycosis, complications and outcome. Neutropenia was defined as absolute neutrophil count ≤1000 cells/mm3 at the time of diagnosis of mucormycosis.
2.3. Statistical analysis
All data were analysed using SPSS Statistics (Version 19.0, IBM Corp.). Descriptive analysis was used for demographic and clinical characteristics. Bivariate analysis was performed on all variables of this study using the chi‐square test.
3. RESULTS
Fifty‐eight patients were evaluated with suspicion of mucormycosis in these canters during the period time; finally, fifteen patients with laboratory‐confirmed COVID‐19 and mucormycosis were included in this study. Median age of patients was 52 years (14–71), and 66% were male. Demographics and clinical characteristic of our patients are summarised in Table 1. The most common symptoms of COVID‐19 were anosmia (60%), fever (33%), cough (27%), dyspnoea (27%) and myalgia (27%). Eight out of 15 patients (53%) had diffuse lung involvement, so were categorised as severe form. The median interval time between the onset of COVID‐19 and first symptoms of mucormycosis was seven days (1–37). All patients had underlying diseases including diabetes mellitus (DM) and hypertension that were the most common comorbidities documented in 13 (87%) and 7 (46%) patients, respectively (Table 2). Seven patients (46%) had received intravenous corticosteroids (either dexamethasone or methylprednisolone) for the management of COVID‐19. One patient had undergone mechanical ventilation support. Two patients (13%) had received interferon, one patient remdesivir and one patient favipiravir along with hydroxychloroquine as anti‐viral treatment. Nine patients (60%) had received nasal O2 support during their COVID‐19 course. Three patients (20%) were neutropenic at the time of admission for mucormycosis, of whom two were suffering from acute myeloid leukaemia (AML) and were taking chemotherapeutic agents (Table 1). In addition, all of the patients showed raised erythrocyte sedimentation rate (ESR) (mean = 81.67, SD = 22.9) and C‐reactive protein (CRP) levels (mean = 81.73, SD = 61.2) during COVID‐19 course. Clinical manifestations of mucormycosis included the following: unilateral periorbital pain and oedema (73%), eyelid ptosis (73%), acute vision loss (73%), proptosis (67%), unilateral facial oedema (60%), cranial nerve palsy (60%), headache (33%), fever (27%), nasal blockage (13%) and ear pain (7%). Based on imaging, intra‐operative endoscopic observation and histopathology evaluation, rhino‐orbital mucormycosis (ROM) was the most frequent form of mucormycosis as evidenced in seven (47%) of COVID‐19 patients, sino‐orbital mucormycosis (SOM) involved 33% of the patients, 13% had isolated orbital involvement, and one patient (7%) was affected by sinonasal mucormycosis (SM). No patient had pulmonary mucormycosis. The most common form of paranasal sinus involvement was pansinusitis. In ten (67%) cases, mucormycosis was extended to skull base spaces. Among patients, 53.3% had pterygopalatine fossa involvement. Cavernous sinus involvement developed in seven cases (46%). Clinical, radiological and histological features in a patient with COVID‐19‐associated mucormycosis are shown in Figure 1. All of the patients were treated with intravenous amphotericin B liposomal (Ambisome Gilead Co.) (IV 5 mg/kg daily for 4–6 weeks), and four (27%) cases took oral posaconazole (Noxafil MSD Co.) (5 ml every 6 h/orally/for 2 weeks) (Combination antifungal agents as a salvage treatment). Three (20%) patients took additional IV Caspofungin (Letocan Nano Alvand Co.) (IV 70 mg stat and 50 mg daily) for 2 weeks (Table 2). The clinical and demographic characteristics of survivors and non‐survivors cases are compared in Table 3. Antifungal combination therapy was significantly associated with better outcome (p = .003). Seven patients (47%) succumbed as the result of mucormycosis. All patients with ROM, SOM and SM underwent sinus debridement, except one patient (case number 5) who had severe lung involvement caused by COVID‐19. Five patients (33%) underwent orbital exenteration, and 2 patients (13%) underwent extensive palatal debridement. At 3 months of follow‐up time, eight patients (53%) had blind frozen eye without exenteration, one patient had frozen seeing eye, and one patient showed improvement of eye symptoms. The all‐cause 30 days of mortality was 47%. No patient died secondary to known COVID‐19 problems.
TABLE 1.
Characteristics of fifteen COVID‐19 patients co‐infected with rhino‐orbital mucormycosis
| Case no. | Gender/Age | Underlying diseases | Severity of COVID‐19 based on Thoracic CT scan | O2 therapy | IV dexamethasone therapy | ICU (day) | Mucormycosis‐associated risk factor | Clinical manifestations of mucormycosis | Clinical form of mucormycosis | Day of Mucormycosis detection after COVID‐19 Dg | Orbital exenteration | Palate exenteration | sinus debridement | Antifungal treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/56 | Diabetes, Hypertension | Severe | Nasal Cannula | Yes | No | Uncontrolled Diabetes, Steroids | Unilateral facial swelling, unilateral periorbital facial pain, orbital inflammation, eyelid oedema, ptosis, proptosis, cranial nerve palsies, acute vision loss | OM | 7 | Yes | No | No | AMB | Alive |
| 2 | M/50 | Diabetes, Hypertension | Severe | Nasal Cannula | Yes | Yes (7) | Uncontrolled Diabetes, Steroids, Neutropenia | Headache, unilateral facial swelling, unilateral periorbital facial pain, orbital inflammation, eyelid oedema, ptosis, proptosis, cranial nerve palsies, acute vision loss | OM | 1 | Yes | No | No | AMB, PSZ | Alive |
| 3 | M/66 | Diabetes, Hypertension | Moderate | Nasal Cannula | No | No | Diabetes, Hypertension | Palate necrosis, orbital inflammation, eyelid oedema, ptosis | ROM | 21 | No | No | Yes | AMB, PSZ | Alive |
| 4 | F/52 | Diabetes, Asthma, Cardiovascular Disease, Hypothyroidism | Severe | Simple Mask | No | No | Uncontrolled Diabetes | Unilateral periorbital facial pain, orbital inflammation, eyelid oedema, ptosis, cranial nerve palsies, acute vision loss | ROM | 21 | Yes | No | Yes | AMB | Alive |
| 5 | F/50 | Diabetes | Moderate | Simple Mask | No | No | Uncontrolled Diabetes | Fever, palate necrosis, unilateral facial swelling, unilateral periorbital facial pain, orbital inflammation, eyelid oedema, cranial nerve palsies, acute vision loss | ROM | 21 | No | No | No | AMB | Death |
| 6 | M/52 | Diabetes | Severe | MV | Yes | Yes (11) | Uncontrolled Diabetes, Steroids | Fever, necrotic nasal, unilateral facial swelling, unilateral periorbital facial pain, Orbital inflammation, eyelid oedema, ptosis, proptosis, cranial nerve palsies, acute vision loss | ROM | 21 | No | No | Yes | AMB, PSZ, CSP | Alive |
| 7 | M/49 | Diabetes | Moderate | Nasal Cannula | No | No | Uncontrolled Diabetes | Headache necrotic nasal, unilateral facial swelling, unilateral periorbital facial pain, orbital inflammation, eyelid oedema, ptosis, proptosis, cranial nerve palsies, acute vision loss, otologic symptoms | ROM | 1 | No | No | Yes | AMB, CSP | Alive |
| 8 | F/49 | Diabetes, Hypertension | Moderate | Nasal Cannula | Yes | Yes (4) | Uncontrolled Diabetes, Steroids | Necrotic nasal, palate necrosis, unilateral facial swelling, unilateral periorbital facial pain, orbital inflammation, eyelid oedema, ptosis, proptosis, cranial nerve palsies, acute vision loss | ROM | 5 | No | Yes | Yes | AMB | Death |
| 9 | M/32 | Haematological Malignancy | Mild | Nasal Cannula | No | No | AML, Chemotherapy, Neutropenia | Fever, headache, necrotic nasal, nasal blockage, unilateral periorbital facial pain, Orbital inflammation, eyelid oedema, proptosis, cranial nerve palsies, | SOM | 7 | No | No | Yes | AMB | Death |
| 10 | M/71 | Diabetes, Hypertension, Cardiovascular Disease | Severe | Simple Mask | Yes | Uncontrolled Diabetes, Steroids | Ptosis, proptosis, acute vision loss | SOM | 14 | No | No | Yes | AMB, PSZ | Alive | |
| 11 | M/55 | Diabetes, Hypertension, Cirrhotic Liver | Severe | NIV | No | Yes (2) | DKA | Necrotic nasal, palate necrosis, unilateral facial swelling, cranial nerve palsies | SOM | 1 | No | Yes | Yes | AMB | Death |
| 12 | M/44 | Diabetes | Severe | Simple Mask | No | Yes (6) | Uncontrolled Diabetes | Necrotic nasal, unilateral periorbital facial pain, orbital inflammation, eyelid oedema, ptosis, proptosis, acute vision loss | SOM | 2 | Yes | No | Yes | AMB | Death |
| 13 | F/70 | Diabetes | Mild | Nasal Cannula | Yes | No | Uncontrolled Diabetes, Steroids | Headache, necrotic nasal, palate necrosis, unilateral facial swelling, unilateral periorbital facial pain, orbital inflammation, eyelid oedema, ptosis, proptosis, acute vision loss, | ROM | 6 | Yes | No | Yes | AMB | Death |
| 14 | M/14 | Haematological Malignancy | Moderate | Nasal Cannula | No | No | AML, Chemotherapy, Neutropenia | Fever, headache, necrotic nasal, nasal blockage, unilateral, facial swelling | SM | 37 | No | No | Yes | AMB, CSP | Alive |
| 15 | M/66 | Diabetes, Hypertension, Asthma, Tuberculosis | Severe | Simple Mask | Yes | No | Uncontrolled Diabetes, Steroids | Necrotic nasal, unilateral periorbital facial pain, Orbital inflammation, eyelid oedema, ptosis, proptosis, acute vision loss | SOM | 18 | No | No | Yes | AMB | Death |
Abbreviations: AMB, amphotericin B; AML, acute myeloid leukaemia; CSP, caspofungin; CT, computed tomography; Dg, diagnosis; DKA, diabetes ketoacidosis; F, female; HE, histopathological examination; IV, intravenous; M, male; MV, mechanical ventilation; NA, not applicable; NIV, non‐invasive ventilation; OM, orbital mucormycosis; PSZ, posaconazole; ROM, rhino‐orbito mucormycosis; SM, sinonasal mucormycosis; SOM, sino‐orbital mucormycosis.
TABLE 2.
Contributing factors, interventions and outcome in patients with COVID‐19‐associated mucormycosis
| Demographic | Gender, Male (n, %) | 10 (66%) |
| Age (Median Years, range) | (14‐71) | |
| Length of hospitalisation (Median days, range) | 30 (3‐90) | |
| Comorbidities (n, %) | DM | 13 (86) |
| Hypertension | 7 (46) | |
| Haematologic malignancies | 2 (13) | |
| Asthma | 2 (13) | |
| Cardiovascular disease | 2 (13) | |
| Hepatic cirrhosis | 1 (6) | |
| Hypothyroidism | 1 (6) | |
| Tuberculosis | 1 (6) | |
| Risk factors (n, %) | Immunosuppressive therapy | 7 (46) |
| Chemotherapy | 2 (13) | |
| Neutropenia | 3 (20) | |
| Ketoacidosis | 1 (6) | |
| Site of mucormycosis infection (n, %) | ROM | 7 (47) |
| SOM | 5 (33) | |
| OM | 2 (13) | |
| SM | 1(7) | |
| Clinical manifestations (n, %) | Nasal congestion or blockage | 2 (13) |
| Fever | 4 (26) | |
| Headache | 5 (33) | |
| Palate necrosis | 5 (33) | |
| Unilateral facial swelling | 9 (60) | |
| Unilateral periorbital facial pain | 11 (73) | |
| Ptosis | 11 (73) | |
| Proptosis | 11 (73) | |
| Acute vision loss | 11 (73) | |
| Cranial nerve palsies | 9 (60) | |
| Otological symptoms | 1 (7) | |
| Laboratory results (Mean ± SD) | WBC | 9391 ± 5886 |
| Lymph count | 1689.3 ± 1879.2 | |
| ESR | 81.6 ± 22.9 | |
| CRP | 81.73 ± 61.2 | |
| HbA1c | 9.86 ± 2.3 | |
| Medication (n, %) | Amphotericin B | 15 (100) |
| Posaconazole | 4 (27) | |
| Caspofungin | 3 (20) | |
| Combined therapy | 6 (40) | |
| Improvement of clinical presentation (n, %) | Improved | 1 (7) |
| Exenterated | 5 (33) | |
| Non‐exenterated blind frozen eye | 8 (53) | |
| Non‐exenterated seeing eye | 1 (7) | |
| Mortality (n, %) | Died | 7 (47) |
| Survived | 8 (53) |
Abbreviations: CRP, C‐reactive protein; DM, diabetes mellitus; ESR, erythrocyte sedimentation rate; lymph count, lymphocyte counts; OM, orbital mucormycosis; ROM, rhino‐orbito mucormycosis; SM, sinonasal mucormycosis; SOM, sino‐orbital mucormycosis; WBC, white blood cells.
FIGURE 1.
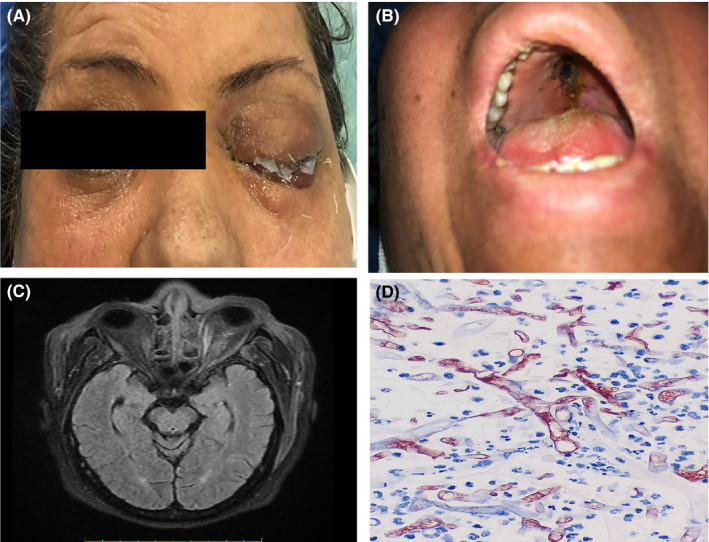
Clinical, radiological and histological features in one of our patients with COVID‐19‐associated mucormycosis. (a) Complete eyelid ptosis, restricted eye movements and no‐light perception in left eye. (b) Palate eschar. (c) Brain MRI, T1‐weighted image after gadolinium injection revealed left ethmoid sinus opacity with mucosal thickening. Enlargement of medial rectus muscle and orbital fat infiltrative pattern. (d) Haematoxylin and eosin (H&E) staining showing broad aseptate right angled hyphae of mucormycosis (1000×magnification)
TABLE 3.
Comparison of demographic and clinical characteristics between survivors and non‐survivors
| Characteristic |
Survivors 8 (%) |
Non‐survivors 7 (%) |
p‐value |
|---|---|---|---|
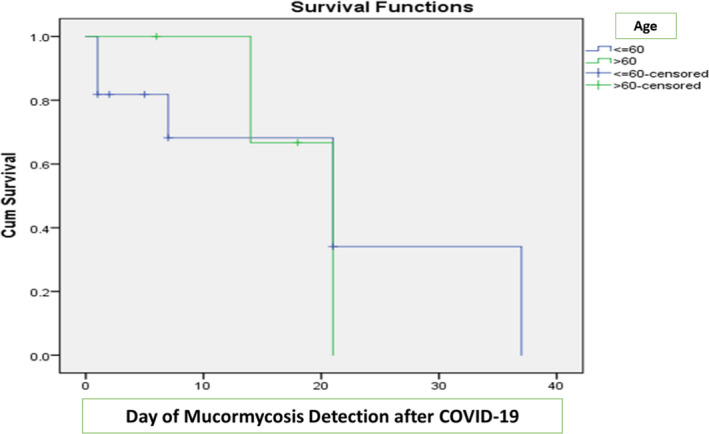
| Age (>60 years) | 2 (25) | 2 ( 28.5) | .876 |
| Sex (male) | 6 (75) | 4 (57.1) | .464 |
| Diabetes Mellitus | 7 (87.5) | 6 (85.7) | .919 |
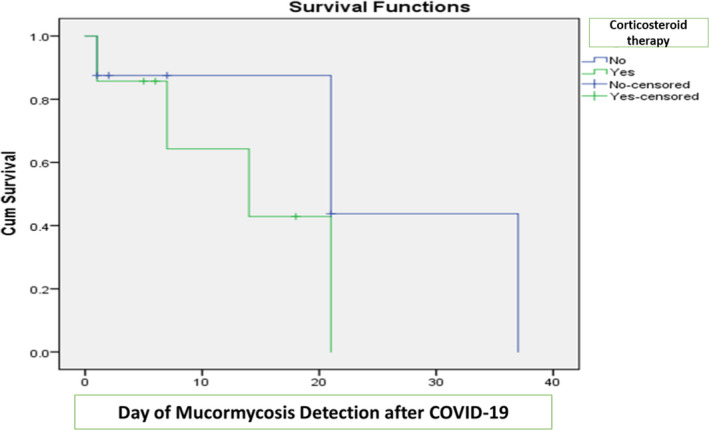
| Corticosteroid therapy | 4 (50) | 3 (42.8) | .782 |
| Chest CT scan severity (severe) | 5 (62.5) | 3 (42.8) | .398 |
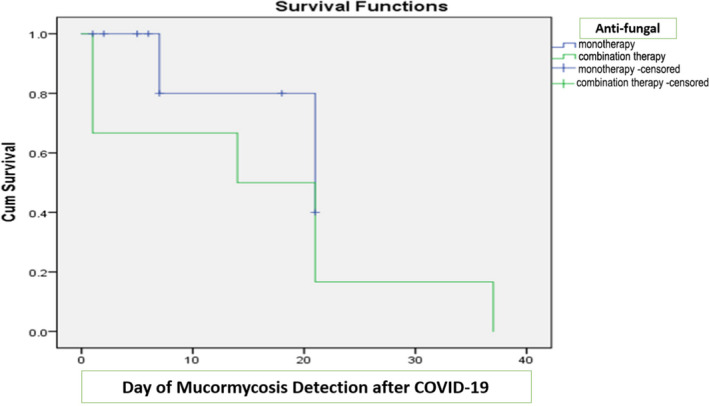
| Antifungal combination therapy | 6 (75) | 0 (0) | .003 |
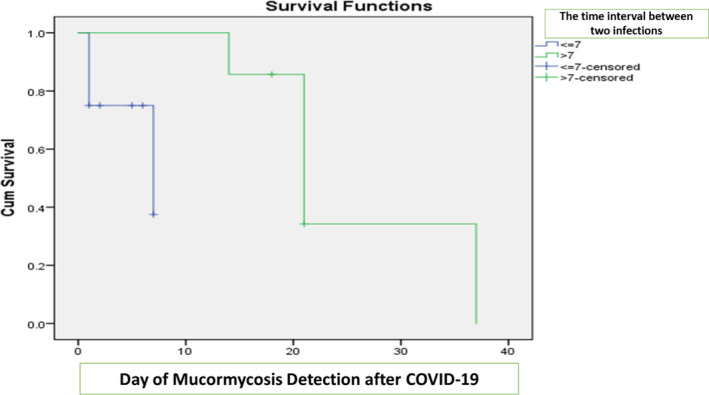
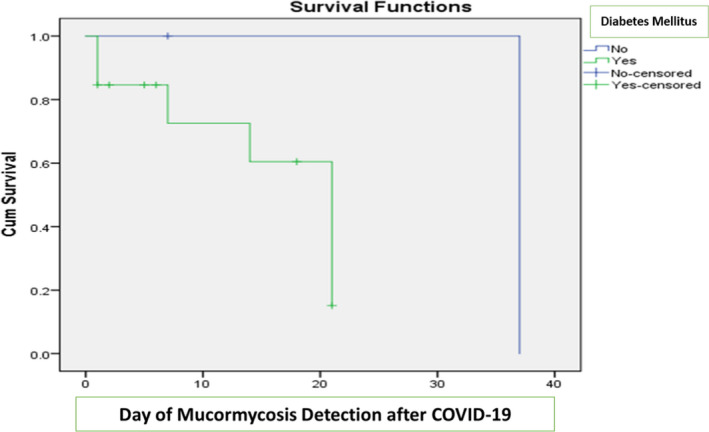
| Day of Mucormycosis Detection after COVID‐19 (>7) | 5 (62.5) | 2 ( 28.5) | .189 |
| ICU admission | 3 (37.5) | 3 (42.8) | .464 |
| O2 therapy (MV/NIV) | 1 (12.5) | 1 (14.2) | .632 |
4. DISCUSSION
Recent reports indicate the association between COVID‐19 and mucormycosis. However, the frequency of COVID‐19‐associated aspergillosis and candidiasis as the most frequent fungal complications in hospitalised COVID‐19 patients has been highlighted in previous studies. 2 , 3 In our previous investigation, 5% of COVID‐19 patients with a history of corticosteroid treatment (47%) and broad‐spectrum antibiotics (92%) developed oropharyngeal candidiasis during hospital admission. 3 White et al. rated invasive fungal infections (IFIs) in 135 COVID‐19 patients. They found a 26.7% incidence of IFIs (commonly aspergillosis (14.1%), or yeast infection, majorly candidiasis (12.6%) among their patients; nonetheless, no case of mucormycosis in their subjects was detected. Corticosteroid therapy and a history of chronic pulmonary disease were the most frequent IFI‐associated risk factors. 17 In this study, we tried to report a series of histology‐proven mucormycosis cases with recent history of COVID‐19. Our study highlights that SARS‐CoV‐2 infection and its related medication may be risk factors for mucormycosis and emphasized the need to monitor high‐risk COVID‐19 patients. 6 The mean interval time between COVID‐19 and mucormycosis was seven days (range: 1–37 days). Consistent with our observation, the mean interval time between diagnosis of COVID‐19 and clinical presentations of oropharyngeal candidiasis and pulmonary aspergillosis was 8 and 11 days, respectively. 3 , 18 Similarly, the result of our literature review of 42 COVID‐19‐associated ROM and ROCM cases demonstrated that mucormycosis was clinically diagnosed at a mean of 12.6 days (range = 0–42 days) after COVID‐19 diagnosis 19 (Table 4). Therefore, based on the available information, it seems that clinicians should be aware of the possible occurrence of mucormycosis during the first to the second week of COVID‐19 in high‐risk patients. 6 , 18 Although the immune responses alleviated and COVID‐19‐associated cytokine storm will be controlled ensuing corticosteroid usage, neutrophil immigration to mucosal surfaces including sinus surfaces will be impaired and vulnerability for developing secondary infections like mucormycosis will be simultaneously increased particularly in patients with DM. 6 , 20 Overall, 47% of our CAM cases were receiving IV corticosteroid for COVID‐19 treatment. Similarly, 40%‐66% of COVID‐19‐associated aspergillosis and 47% of COVID‐19‐associated oropharyngeal candidiasis had a history of steroid therapy. 2 , 3 Of 42 COVID‐19‐associated ROM and rhino‐orbito‐cerebral mucormycosis (ROCM) previously reported cases, 36 cases (85.7%) had a history of systemic corticosteroid treatment prior to mucormycosis diagnosis (Table 4). Comparatively, Hoenigl et al. 19 found that 75% of 80 COVID‐19 patients with mucormycosis had been treated with systemic corticosteroids which in 80% of them, systemic corticosteroids had been started prior to the diagnosis of mucormycosis that supports our finding. It seems reasonable to apply systemic corticosteroids cautiously in patients with COVID‐19. 6 As evidenced previously, uncontrolled DM documented as the prevailing risk factor implicated in mucormycosis development. 21 , 22 In our study, 87% of CAM cases had poorly controlled DM and one patient had DKA when mucormycosis diagnosed. The data were found to be consistent with the findings of our literature review regarding COVID‐19‐associated ROM and ROCM (38/42, 90%) (Table 4) and Hoenigl et al's review (66/80, 82.5%). 19 Geographically, diabetes was even more frequently observed as risk factor in cases from India (32/34, 94%) and USA (3/3, 100%) vs 3/5 (60%) among COVID‐19‐associated ROM and ROCM cases reported from other countries (Table 4). Not only the combination of corticosteroid therapy and diabetes mellitus can result in poorly controlled status of diabetes and synergistically paralyse the function of innate immunity but also corticosteroid‐induced diabetes may occurr in healthy individuals who are receiving long term steroid therapy, thereby augmenting the risk of mucormycosis in a susceptible individual. 6 Meanwhile, it is supposed that ketosis or ketoacidosis and induced diabetic ketoacidosis may be caused by COVID‐19 in those with diabetes. 23 , 24 In addition, the possible role of blood acidosis in a severe form of COVID‐19 and elevated levels of serum ferritin cannot be ignored for mucormycosis susceptibility. 4 , 6 , 20 The presence of DM along with other COVID‐19‐associated medications and complications could be important risk factors for mucormycosis. Two of our subjects (13.3%) were undergoing chemotherapy due to acute myeloid leukaemia (AML) and had profound neutropenia (<100 cells/mm3). Regardless of COVID‐19 status and receiving corticosteroids, as affirmed by our data, patients with profound neutropenia and those suffering from acute haematological malignancies (HMs) are at high risk to develop mucormycosis. 20 Besides, Hoenigl et al. equally noted that 5/80 patients (6.2%) were suffering from HMs. However, none of 42 COVID‐19‐associated ROM and ROCM previously reported cases were suffering from either neutropenia or HM (Table 4). Although ROCM is the commonest manifestation of mucormycosis in patients with poorly controlled diabetes, the lung is the more frequent site of involvement in patients with HMs. 21 , 25 The early manifestation of mucormycosis in 73% of our patients was orbital apex syndrome. This shows a rapid progression of the disease to orbit at presentation. Nonetheless, no case of ROCM was observed in our investigation that was not in agreement with the observation of Hoenigl et al. 19 reporting rhino‐orbital‐cerebral infection as the most commonly presented form of mucormycosis in COVID‐19 patients (59/80, 74%). In the present study, despite antifungal treatment and surgical measures, the mortality rate was as high as 47%. Given the acuteness and aggressiveness of the infection, a timely diagnosis for prompt antifungal therapy is highly recommended in order to decrease the rate of mortality. 24 Interestingly, 100% of our patients who received combined antifungal treatment survived (Appendix 1). More so, 75% of 8 COVID‐19‐associated ROM and ROCM previously reported cases who received combined antifungal treatment survived (Table 4). Combined antifungal treatment may be associated with improved response and a higher rate of survival (p‐value: .003). Limitations of this study include limited sample size preventing a subgroup analysis, absence of a control group for comparing clinical, imaging features, therapeutic interventions, comparison of all COVID‐19 clinical and laboratory factors between those affected and not affected by mucormycosis. The role of combined antifungal treatment and the effect of disease stage on prognosis is the subject of future studies.
TABLE 4.
Clinical characteristics, risk factors, treatment and outcome of reported COVID‐19‐associated mucormycosis
| Author/year/References | Country | Age/gender | Outcome | Surgical intervention | Antifungal treatment | Clinical form of mucormycosis | Interval between diagnosis of COVID‐19 and mucormycosis occurrence (days) | Mucormycosis‐associated risk factor | Local/systemic corticosteroid therapy | O2 supplementation | Underlying Conditions |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mehta S/2020 26 | India | M/60 | Died | Yes | AMB | ROCM | 12 | Uncontrolled diabetes, Steroid for COVID‐19 | Yes‐ IV methylprednisolone and dexamethasone | NIV, MV | Diabetes |
| Sen et al./2021 27 | India | M/46 | Survived | Yes | AMB, VRZ, PSZ | ROCM | 0 | Uncontrolled diabetes, Steroid for COVID‐19 | No | NA | Diabetes |
| M/61 | Survived | Yes | AMB, PSZ | ROM | 17 | Uncontrolled diabetes, Steroid for COVID‐19 | Yes‐ IV methylprednisolone, oral prednisolone | NA | Diabetes, HTN | ||
| M/74* | Survived | Yes | AMB, PSZ | ROCM | 30 | Diabetes, Steroid for COVID‐19 | Yes‐ IV dexamethasone, oral prednisolone | NA | Diabetes, HTN, CAD | ||
| M/73 | Survived | Yes | AMB, PSZ | ROCM | 14 | Uncontrolled diabetes, Steroid for COVID‐19 | Yes‐ oral prednisolone | NA | Diabetes | ||
| M/62 | Survived | Yes | AMB, PSZ | ROCM | 42 | Uncontrolled diabetes, Steroid for COVID‐19 | Yes‐ IV dexamethasone | NA | Diabetes, HTN | ||
| M/62 | Survived | Yes | AMB | ROCM | 3 | Uncontrolled diabetes, Steroid for COVID‐19 | Yes‐ IV dexamethasone | NA | Diabetes, CAD | ||
| Sarkar et al./2021 28 | India | 10 cases**/M (n = 8), F (n = 2)/45.5 | Survived (n = 6), Died (n = 4) | Yes (n = 7) | AMB (n = 10) | ROM (n = 9), ROCM (n = 1) | NA | Diabetes (n = 1), DKA (n = 9), steroid for COVID‐19 (n = 10) | Yes‐ IV dexamethasone (n = 10) | MV (n = 9) |
Diabetes (n = 10) |
| Moorthy et al./2021 29 | India | 17 cases/M (n = 15), F (n = 2)/55 | Survived (n = 11) | Yes | AMB | SM (n = 3), ROM (n = 6), ROCM (n = 5), RCM (n = 3) | NA | Uncontrolled diabetes (n = 15) | Yes (n = 15) | NA | Diabetes (n = 15) |
| Died (n = 6) | |||||||||||
| Karimi Galougahi et al./2021 30 | Iran | F/61 | Survived | Yes | Systemic antifungals | ROM | 21 | Glucocorticoid‐induced diabetes, Steroid for COVID‐19 | Yes | NA | No |
| Veisi et al./2021 31 | Iran | F/40 | Died | Yes | AMB | ROCM | 15 | Steroid for COVID‐19 | Yes‐ IV dexamethasone | NA | No |
| M/54 | Survived | Yes | AMB, PSZ | ROM | 7 | Diabetes, Steroid for COVID‐19 | Yes‐ IV dexamethasone | NIV | Diabetes | ||
| Werthman/2020 32 | USA | F/33 | Died | Yes | AMB | ROCM | 2 | DKA | No | NA | Diabetes, Asthma, HTN |
| Mekonnen/2020 33 | USA | M/60 | Died | Yes | AMB, CSP, PSZ | ROM | 7 | Uncontrolled diabetes, Steroid for COVID‐19 | Yes‐ IV dexamethasone | MV | Diabetes, Asthma, HTN, Hyperlipidaemia |
| Dallalzadeh et al./2021 34 | USA | M/48 | Died | No | AMB/ISZ | ROM | 6 | Diabetes, Steroid for COVID‐19 | Yes‐IV dexamethasone | MV | Diabetes |
| Hanley/2020 35 | UK | M/22 | Died | No | No | Disseminated (involving the hilar lymph nodes, heart, brain, and kidney)/ | NA | Steroid for COVID‐19 | Yes | MV | Pancreatitis |
| Waizel‐Haiat et al./2021 36 | Mexico | F/24 | Died | No | AMB | ROM | 1 | DKA | NA | MV | Obesity, Diabetes |
Abbreviations: AMB, amphotericin B; CAD, coronary artery disease; CSP, caspofungin; DKA, diabetes ketoacidosis; F, female; HTN, hypertension; ISZ, isavuconazole; ISZ, isavuconazole; IV, intravenous; M, male; NA, not applicable (not mentioned in the article); NIV, non‐invasive ventilation; PSZ, posaconazole; RCM, rhino‐cerebral mucormycosis; ROCM, rhino‐orbito‐cerebral mucormycosis; ROM, rhino‐orbital mucormycosis; SM, sinonasal mucormycosis; VRZ, voriconazole.
As per the EORTC‐MSG criteria, the case was categorised as possible mucormycosis.
As per the EORTC‐MSG criteria, three patients were defined to have possible mucormycosis.
In conclusion, the findings of this study showed that clinicians should be more alert about mucormycosis especially during the first to second week after COVID‐19 in diabetic and immunocompromised patients. Poor control of DM seems to be important predisposing factor.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Farzad Pakdel : Conceptualization (equal); Data curation (equal); Investigation (equal); Methodology (equal); Resources (equal); Supervision (lead); Validation (equal); Visualization (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Kazem Ahmadikia: Data curation (equal); Methodology (equal); Resources (equal); Software (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Mohammadreza Salehi: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Resources (equal); Supervision (equal); Validation (equal); Visualization (equal); Writing‐original draft (equal); Writing‐review & editing (equal). Azin Tabari: Investigation (equal); Methodology (equal); Resources (equal); Visualization (equal). Rozita Jafari: Investigation (equal); Resources (equal); Visualization (equal); Writing‐review & editing (equal). Golfam Mehrparvar: Investigation (equal); Software (equal); Visualization (equal); Writing‐review & editing (equal). Yasaman Rezaie: Formal analysis (equal); Investigation (equal); Methodology (equal); Resources (equal); Software (equal); Writing‐original draft (equal). Shahin Rajaeih: Investigation (equal); Methodology (equal); Resources (equal). Neda Alijani: Investigation (equal); Methodology (equal); Resources (equal). Aleksandra Barac: Validation (equal); Writing‐review & editing (equal). Alireza Abdollahi: Investigation (equal); Methodology (equal); Resources (equal). sadegh Khodavaisy: Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Resources (equal); Software (equal); Validation (equal); Visualization (equal); Writing‐original draft (equal); Writing‐review & editing (equal).
ETHICAL APPROVAL
This study approved by the ethics committee of Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.VCR.REC.1399.152). To ensure anonymity, details that might disclose the identity of the subject under the study were not included. Written informed consent was obtained from the patient prior to being included in the study.
CODE AVAILABILITY
All data were analysed using SPSS Statistics (Version 19.0, IBM Corp.).
ACKNOWLEDGEMENTS
This study has been funded and supported by Tehran University of Medical Sciences (TUMS); Grant no. 1400‐1‐99‐51467. Authors would like to thank Ms Elham Roshan, Ms Marjan Marvi and Mr Kosali for their dedication for scheduling timely visit and surgical appointments and the patients' postoperative care. We also are so appreciating Mr Mostafa Salehi for data analysis.
APPENDIX 1. Kaplan‐Meier curves for survivors
Pakdel F, Ahmadikia K, Salehi M, et al. Mucormycosis in patients with COVID‐19: A cross‐sectional descriptive multicentre study from Iran. Mycoses. 2021;64:1238–1252. 10.1111/myc.13334
Funding information
This study has been funded and supported by Tehran University of Medical Sciences (TUMS); Grant no. 99‐1‐252‐47469
DATA AVAILABILITY STATEMENT
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Salehi M, Ahmadikia K, Badali H, Khodavaisy S. Opportunistic fungal infections in the epidemic area of COVID‐19: a clinical and diagnostic perspective from Iran. Mycopathologia. 2020;185(4):607‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID‐19. Lancet Respir Med. 2020;8(6):e48‐e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salehi M, Ahmadikia K, Mahmoudi S, et al. Oropharyngeal candidiasis in hospitalised COVID‐19 patients from Iran: species identification and antifungal susceptibility pattern. Mycoses. 2020;63(8):771‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guan W‐J, Ni Z‐Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahmadikia K, Hashemi SJ, Khodavaisy S, et al. The double‐edged sword of systemic corticosteroid therapy in viral pneumonia: a case report and comparative review of influenza‐associated mucormycosis versus COVID‐19 associated mucormycosis. Mycoses. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lai C‐C, Wang C‐Y, Hsueh P‐R. Co‐infections among patients with COVID‐19: the need for combination therapy with non‐anti‐SARS‐CoV‐2 agents? Journal of Microbiology, Immunology and Infection. 2020;53(4):505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu J, Zheng X, Tong Q, et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‐CoV, MERS‐CoV, and 2019‐nCoV. J Med Virol. 2020;92(5):491‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vanderbeke L, Spriet I, Breynaert C, Rijnders BJ, Verweij PE, Wauters J. Invasive pulmonary aspergillosis complicating severe influenza: epidemiology, diagnosis and treatment. Curr Opin Infect Dis. 2018;31(6):471‐480. [DOI] [PubMed] [Google Scholar]
- 10. Li X, Zeng X, Liu B, Yu Y. COVID‐19 infection presenting with CT halo sign. Radiol Cardiothorac Imaging. 2020;2(1):e200026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al‐Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID‐19) pandemic: an international collaborative group. Oncologist. 2020;25(6):e936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kutikov A, Weinberg DS, Edelman MJ, Horwitz EM, Uzzo RG, Fisher RI. A war on two fronts: cancer care in the time of COVID‐19. Ann Intern Med. 2020;172(11):756–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Silva LN, de Mello TP, de Souza Ramos L, Branquinha MH, Roudbary M, dos Santos ALS. Fungal infections in COVID‐19‐positive patients: a lack of optimal treatment options. Curr Top Med Chem. 2020;20(22):1951‐1957. [DOI] [PubMed] [Google Scholar]
- 14. Antinori S, Bonazzetti C, Gubertini G, et al. Tocilizumab for cytokine storm syndrome in COVID‐19 pneumonia: an increased risk for candidemia? Autoimmun Rev. 2020;19(7):102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richardson M. The ecology of the Zygomycetes and its impact on environmental exposure. Clin Microbiol Infect. 2009;15:2‐9. [DOI] [PubMed] [Google Scholar]
- 16. WHO . Clinical Management of COVID‐19: Interim Guidance, 27 May 2020. World Health Organization; 2020. https://apps.who.int/iris/handle/10665/332196(2020). [Google Scholar]
- 17. White PL, Dhillon R, Cordey A, et al. A National strategy to diagnose coronavirus disease 2019–associated invasive fungal disease in the intensive care unit. Clin Infect Dis. 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dupont D, Menotti J, Turc J, et al. Pulmonary aspergillosis in critically ill patients with Coronavirus Disease 2019 (COVID‐19). Med Mycol. 2021;59(1):110‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hoenigl M, Seidel D, Carvalho A, et al. The emergence of COVID‐19 associated mucormycosis: analysis of cases from 18 countries. 2021. [DOI] [PMC free article] [PubMed]
- 20. Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634‐653. [DOI] [PubMed] [Google Scholar]
- 22. Cornely OA, Alastruey‐Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405‐e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID‐19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22(10):1935‐1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skiada A, Lanternier F, Groll AH, et al. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica. 2013;98(4):492‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robin C, Alanio A, Cordonnier C. Mucormycosis: a new concern in the transplant ward? Curr Opin Hematol. 2014;21(6):482‐490. [DOI] [PubMed] [Google Scholar]
- 26. Mehta S, Pandey A. Rhino‐orbital mucormycosis associated with COVID‐19. Cureus. 2020;12(9):e10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sen M, Lahane S, Lahane TP, Parekh R, Honavar SG. Mucor in a viral land: a tale of two pathogens. Indian J Ophthalmol. 2021;69(2):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sarkar S, Gokhale T, Choudhury SS, Deb AK. COVID‐19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69(4):1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moorthy A, Gaikwad R, Krishna S, et al. SARS‐CoV‐2, uncontrolled diabetes and corticosteroids—an unholy trinity in invasive fungal infections of the maxillofacial region? a retrospective, multi‐centric analysis. J Maxillofac Oral Surg. 2021:1‐8. https://link.springer.com/article/10.1007/s12663‐021‐01532‐1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Karimi‐Galougahi M, Arastou S, Haseli S. Fulminant mucormycosis complicating coronavirus disease 2019 (COVID‐19). Int Forum Allergy Rhinol. 2021;11(6):1029‐1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Veisi A, Bagheri A, Eshaghi M, Rikhtehgar MH, Rezaei Kanavi M, Farjad R. Rhino‐orbital mucormycosis during steroid therapy in COVID‐19 patients: a case report. Eur J Ophthalmol. 2021. 10.1177/11206721211009450. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Werthman‐Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID‐19. Am J Emerg Med. 2021;42(264):264.e5‐264.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mekonnen ZK, Ashraf DC, Jankowski T, et al. Acute invasive rhino‐orbital mucormycosis in a patient with COVID‐19‐associated acute respiratory distress syndrome. Ophthalmic Plast Reconstr Surg. 2021;37(2):e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dallalzadeh LO, Ozzello DJ, Liu CY, Kikkawa DO, Korn BS. Secondary infection with rhino‐orbital cerebral mucormycosis associated with COVID‐19. Orbit. 2021:1‐4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35. Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID‐19: a post‐mortem study. Lancet Microbe. 2020;1(6):e245‐e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Waizel‐Haiat S, Guerrero‐Paz JA, Sanchez‐Hurtado L, Calleja‐Alarcon S, Romero‐Gutierrez L. A case of fatal rhino‐orbital mucormycosis associated with new onset diabetic ketoacidosis and COVID‐19. Cureus. 2021;13(2):e13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.


