Abstract
Background
While convalescent plasma (CP) may benefit patients with COVID‐19, fundamental questions remain regarding its efficacy, including the components of CP that may contribute to its therapeutic effect. Most current serological evaluation of CP relies on examination of total immunoglobulin or IgG‐specific anti‐SARS‐CoV‐2 antibody levels. However, IgA antibodies, which also circulate and are secreted along the respiratory mucosa, represent a relatively uncharacterized component of CP.
Study design and methods
Residual samples from patients and CP donors were assessed for IgM, IgG, and IgA anti‐SARS‐CoV‐2 antibody titers against the receptor‐binding domain responsible for viral entry. Symptom onset was obtained by chart review.
Results
Increased IgA anti‐SARS‐CoV‐2 antibody levels correlated with clinical improvement and viral clearance in an infant with COVID‐19, prompting a broader examination of IgA levels among CP donors and hospitalized patients. Significant heterogeneity in IgA levels was observed among CP donors, which correlated weakly with IgG levels or the results of a commonly employed serological test. Unlike IgG and IgM, IgA levels were also more likely to be variable in hospitalized patients and this variability persisted in some patients >14 days following symptom onset. IgA levels were also less likely to be sustained than IgG levels following subsequent CP donation.
Conclusions
IgA levels can be very heterogenous among CP donors and hospitalized patients and do not necessarily correlate with commonly employed testing platforms. Examining isotype levels in CP and COVID‐19 patients may allow for a tailored approach when seeking to fill specific gaps in humoral immunity.
Keywords: antibody isotype, convalescent plasma, COVID‐19, IgA, serology
1. INTRODUCTION
A multi‐center study including 35,322 patients from 2807 acute care facilities by the US expanded access program COVID‐19 Plasma Consortium found reductions in 7 and 30 days mortality when convalescent plasma (CP) with high levels of SARS‐CoV‐2‐specific IgG antibodies was delivered early during infection. 1 High‐quality follow‐up trials have recently confirmed the overall findings of efficacy in this analysis. 2 , 3 In addition to timing, concentration, and specificity, antibody isotypes also dictate potency, function, and localization of the humoral immune response to viral pathogens. 4
Due to the selective secretion of dimeric IgA across mucosal barriers, 5 these antibodies could provide an important benefit or immunomodulatory capacity when targeting respiratory pathogens. 6 , 7 Circulating dimeric and monomeric IgA may also mediate isotype‐specific function independent of localization. 8 Monomeric IgA found in the serum lacks the secretory chain of dimeric IgA allowing binding to the Fcα‐receptor 1 (FcαR1), engagement of which can contribute to inflammatory programs in myeloid cells. 9 There is also experimental evidence for an anti‐inflammatory role for monomeric IgA interactions with FcαR1 10 and for therapeutic administration of IgA in autoimmune models. 11 Furthermore, these effects are likely to be dependent on subclass (IgA1 vs. IgA2) and antibody glycosylation as well as concentration and antigen specificity. 11 The secretory component of dimeric IgA facilitates interactions with the polymeric immunoglobulin receptor, 5 allowing dimeric IgA to be secreted across epithelial barriers and provide direct humoral immunity at the mucosal surface. 6 , 7 Together, these studies suggest that monomeric and dimeric IgA can play multiple roles and strongly support further characterization in CP and other therapeutic blood products.
If the goal of CP therapy is to deliver virus‐specific antibodies to the site of infection, 12 assessment of IgA anti‐SARS CoV‐2 antibody levels in CP may be an important step in defining correlates of CP efficacy in larger studies. Despite the potential contribution of IgA anti‐SARS‐CoV‐2 antibodies in CP efficacy, the relative distribution of these antibodies among CP donors and hospitalized patients remains incompletely understood. 13 Prior to the development of serological tests, early efforts to procure CP understandably relied on donors who recovered from PCR confirmed infection in the absence of pre‐transfusion serological assessment. 14 , 15 , 16 The early inability to characterize antibody levels also made it difficult to determine antibody levels in patients prior to transfusion and therefore define potential gaps in humoral immunity that may benefit from this therapy. Even following the development of tests capable of assessing total or IgG‐specific anti‐SARS‐CoV‐2 antibody levels or neutralizing titers, the majority of these platforms do not specifically evaluate IgA. Characterization of units and responses to CP have been performed in recent months, 17 , 18 but relative levels of class‐specific components remain incompletely understood. As IgA anti‐SARS‐CoV‐2 antibody levels may reflect an under characterized yet important variable when seeking to establish CP therapeutic efficacy, we sought to define these levels in CP donors and hospitalized patients.
2. METHODS
2.1. Anti‐SARS CoV‐2 antibody evaluation
SARS‐CoV‐2 PCR‐confirmed patients were initially identified based on hospital wide SARS‐CoV‐2 PCR testing results. Residual plasma samples (primarily in sodium citrate as an anticoagulant) from clinical laboratory tests were collected as “discarded samples,” aliquoted and stored at −80°C prior to analysis for antibody levels. Plasma obtained at the time of CP unit collection was similarly aliquoted and stored at −80°C prior to antibody evaluation. Purified recombinant receptor‐binding domain (RBD) from the SARS‐CoV‐2 was generated as recently outlined and used as the target. 19 , 20 Briefly, 1 μg/ml of purified recombinant RBD in phosphate buffer saline (PBS) was incubated overnight at 4°C or at 37°C for 1 h. Plates were then washed 3x with 0.5% T20 in PBS (PBST) and blocked for 30 min at room temperature (RT) in ELISA buffer (1% Bovine serum albumin BSA, 0.2% T20 in PBS). Starting at 1:50, 1:3 serial dilutions were then analyzed for isotype‐specific anti‐RBD antibody levels using anti‐human IgA (Southern biotek, Birmingham, AL), IgG or IgM (Invitrogen, Carlsbad, CA) antibodies, followed by O‐phenylenediamine dihydrochloride for development and a BIOTEK plate reader at 492 nm. For analysis of CP units given to the patient presented in the clinical case, an aliquot of each CP unit was assessed. For analysis of CP donors in general, the same strategy employed by LifeSouth was adopted to facilitate a direct comparison of serology assay performance characteristics. In LifeSouth's protocol, an additional blood draw at the time of CP collection is used for SARS‐CoV‐2 antibody testing. An aliquot of the same sample tested for SARS‐CoV‐2 antibodies at LifeSouth (Ortho VITROS) was used for assessing IgA, IgG, and IgM as outlined above. All data generated on the Ortho VITROS (Ortho clinical diagnostics, Raritan, NJ) were performed by LifeSouth Donation Services.
2.2. Chart review
Chart reviewers were blind to the ELISA results at the time of review. Patient information was entered into a REDCap® database. Symptom onset dates were determined using defined criteria, where at least one of the following needed to be reported as a new symptom on the estimated date of onset (cough, shortness of breath or difficulty breathing, fever [including subjective fever], chills, muscle pain, headache, sore throat, loss of taste or smell, rash, or diarrhea). To enhance symptom onset date reliability, all dates were independently checked by at least one additional chart reviewer.
2.3. Study approval and ethical statement
Sample collection and chart review was accomplished under the approval of the Institutional Review board (IRB #00022371).
3. RESULTS
3.1. CP therapy in an infant with Trisomy 21 and COVID‐19
To examine the possibility that IgA levels may influence CP efficacy, we first explored IgA antibody levels in samples acquired before and after transfusion of a recently reported infant where significant clinical improvement and evidence of viral clearance were observed shortly after CP therapy. 21 This infant was a 3.1 kg term 9‐week‐old female with a history significant for Trisomy 21 and an unrepaired balanced complete atrioventricular canal defect who presented to the hospital with respiratory failure initially thought to be due to decompensated heart failure. Despite stabilization with milrinone, intravenous diuretics and bilevel noninvasive positive pressure ventilation, by hospital day 12 she developed acute respiratory failure and her heart failure once again decompensated. Nasopharyngeal swab real‐time PCR testing was positive for SARS‐CoV‐2 and additional testing of a residual sample collected for a general respiratory panel on hospital day 5 was also positive for SARS‐CoV‐2. Given the patient's underlying congenital heart disease and respiratory compromise in the face of COVID‐19, efforts were taken to eradicate the virus. Initial management of the viral infection was essential given that significant post‐operative complications are associated with atrioventricular canal defect repair in patients with pre‐existing respiratory infection. 22 , 23 , 24
In an effort to clear the virus, a 14‐day trial of remdesivir was initiated. However, despite this intervention, the patient remained PCR positive on repeat SARS‐CoV‐2 testing from hospital day 5 to 25 (Figure 1(A)). While very little was known regarding the effectiveness of CP in COVID‐19 patients, especially infants with congenital heart disease, the empirical use of CP was considered to treat the ongoing viral infection and prepare the patient for atrioventricular canal repair. To this end, the patient received two doses of CP (10 ml/kg/dose), a day apart, from the same CP donor (donor 1). Despite remaining SARS‐CoV‐2 PCR positive following each CP infusion, SARS‐CoV‐2 PCR testing results were negative for the first time since hospital day 5 the day following the second CP dose (hospital day 30) (Figure 1(A)). In addition, the patient appeared to improve clinically as evidenced by reduced ventilator support. As the sustainability of this initial response was uncertain, the patient received two additional doses of CP on hospital days 31 and 32 from a different donor (donor 2) (Figure 1(A)). While a positive SARS‐CoV‐2 PCR test occurred following the second CP infusion from donor 2, the patient primarily remained negative over the next week, with only a few intermittent positive SARS‐CoV‐2 PCR results. More importantly, the patient's clinical status continued to improve. She was extubated on hospital day 34 and eventually underwent successful atrioventricular canal defect repair.
FIGURE 1.
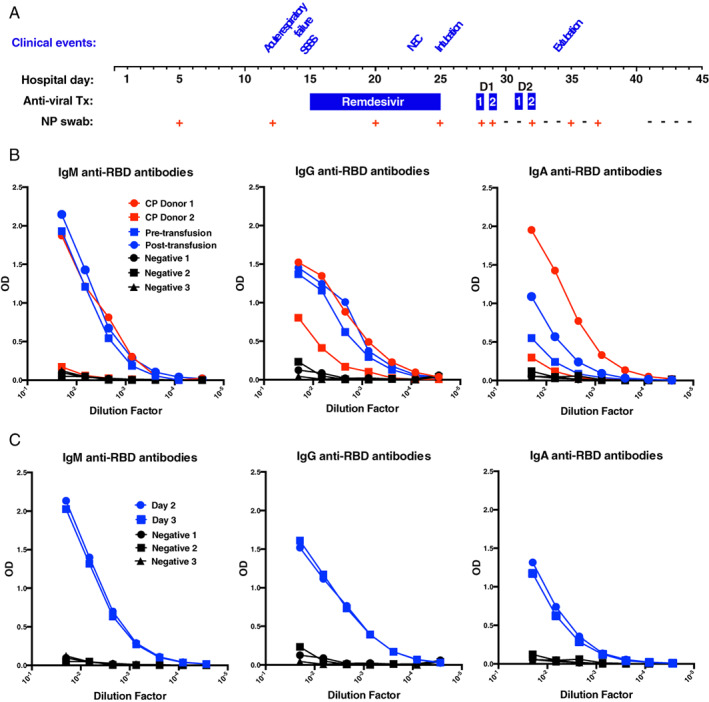
Clinical course and SARS‐CoV‐2 antibody changes after CP for COVID‐19 in an infant with trisomy 21. (A) Schematic representation of the COVID‐19 clinical course of an infant infected with SARS‐CoV‐2 who received CP therapy. (B) Levels of SARS‐CoV‐2 RBD‐specific antibody were assessed by limiting dilution pre‐ and post‐infusion of serologically characterized convalescent plasma (CP) units. CP donor 1 = convalescent plasma donor 1 (from whom the first two doses of CP were derived); CP donor 2 = convalescent plasma donor 2 (from whom the third and fourth doses of CP were derived); pre‐transfusion = sample taken prior to CP therapy. Post‐transfusion = sample taken after the first dose of CP. Negative 1, 2, and 3 = samples taken from three distinct individuals negative for COVID‐19. (C) Levels of class‐specific antibodies were similarly monitored on days 2 and 3 following the first dose of CP. D1, donor 1; D2, donor 2; NEC, necrotizing enterocolitis; NP swab, nasopharyngeal swab PCR result; RBD, receptor‐binding domain; SSSS, staphlococcus scalded skin syndrome
At the time of CP therapy, CP units or patients were not routinely evaluated for anti‐SARS‐CoV‐2 antibodies prior to transfusion. However, we were able to retrospectively perform limiting dilution analysis using SARS‐CoV‐2 ELISAs for antiviral IgG, IgM, and IgA on residual samples obtained pre‐ and post‐transfusion from the infant as well as samples from the CP donor units themselves. IgM, IgG, and IgA antibody levels against the RBD of SARS‐CoV‐2 (antibodies that correlate strongly with neutralizing activity 19 ) were found to be high in the first CP unit transfused, while the second unit of CP exhibited low antibody levels overall (Figure 1(B)). As samples were available prior to and following the first CP unit, anti‐SARS‐CoV‐2 antibody levels were next evaluated in the patient. Despite having high IgG antibody levels, the first CP unit failed to significantly increase IgG or IgM antibody levels in this patient, perhaps due to the higher titer IgG and IgM antibodies already present (Figure 1(B, C)). In contrast, IgA antibody levels increased significantly following CP transfusion (Figure 1(B,C)). While these data do not demonstrate that IgA alone supported viral clearance, the isolated increase in IgA anti‐SARS‐CoV‐2 antibodies following CP transfusion coupled with the proximity of viral clearance and clinical improvement to initial CP therapy suggests that increases in IgA anti‐SARS‐CoV‐2 antibodies could be associated with infection resolution.
3.2. Isotype‐specific SARS‐CoV‐2 serology in CP units
Given the variability in antibody levels between the two CP units used to treat this patient and the possible association of CP‐induced increases in IgA anti‐SARS‐CoV‐2 levels and clinical improvement in this patient, we next determined IgM, IgG, and IgA levels over a larger pool of CP donor units (n = 220). Significant variability was observed in IgM, IgG, and IgA levels among CP donors. To determine whether IgA levels correlate with IgG or IgM levels, we first assessed IgG and IgM levels based on increasing IgA. Many units with high levels of IgA likewise exhibited high levels of IgG and IgM, suggesting that examination of IgG alone may suffice when seeking to characterize the overall repertoire of anti‐SARS‐CoV‐2 isotypes (Figure 2(B)). However, differences between IgA and IgG levels were noted and when units were instead stratified based on IgA‐negative results, many units were strongly positive for IgG antibodies despite the absence of IgA (Figure 2(A)). Similarly, while general correlations across all donor IgM, IgG, and IgA levels were observed (Figure 2(B)), significant variation existed, suggesting that in addition to total antibody levels, the composition of antibody isotypes can vary between CP units.
FIGURE 2.
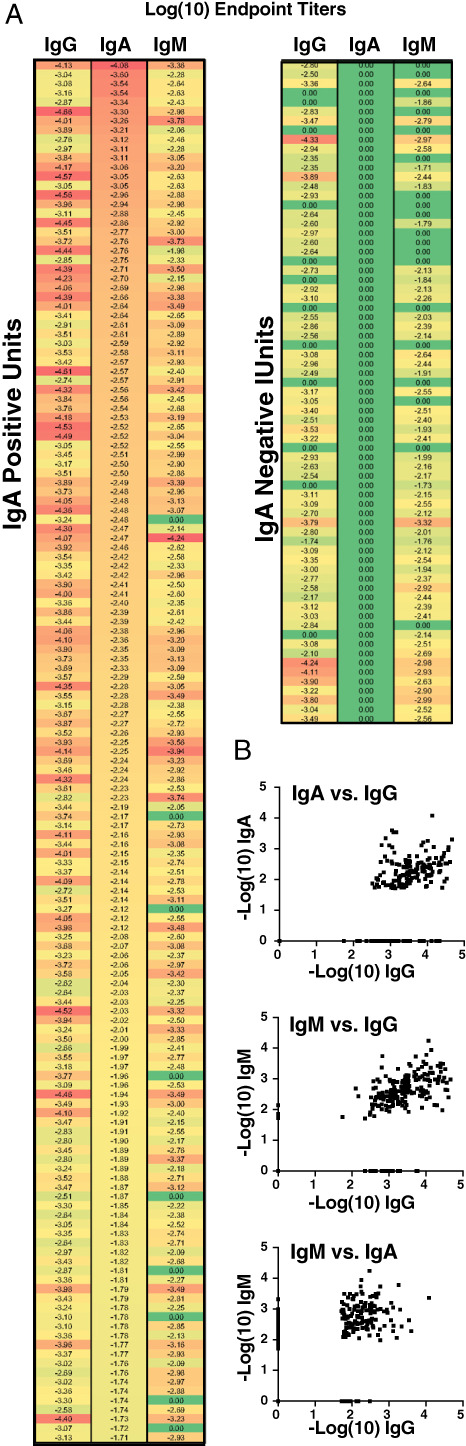
Receptor‐binding‐domain‐specific IgA levels in COVID‐19 convalescent plasma (CP) units are variable. (A) Heatmap presenting our assessment of 220 COVID‐19 CP units by antibody class‐specific SARS‐CoV‐2 IgG, IgA, and IgM, sorted and partitioned by presence of detectable circulating IgA (N = 71 units or 32% not detected). (B) Association between IgG, IgA, and IgM levels in units of CP (N = 220)
3.3. Population level longitudinal analysis of SAR‐CoV‐2 RBD antibodies in hospitalized patients over time
As differences between IgG and IgA were also observed for our patient, we next examined whether similar differences in isotypes exist among other COVID‐19 hospitalized patients (n = 201 samples, Figure 3(A)). Although lower levels of all three antibody isotypes were observed within the first 10 days following symptom onset, patients nearly uniformly possessed high IgM and IgG anti‐RBD antibody levels by 14 days post‐symptom onset (Figure 3(A)). In contrast, some patients continued to exhibit low levels of IgA despite evidence of IgM and IgG seroconversion.
FIGURE 3.
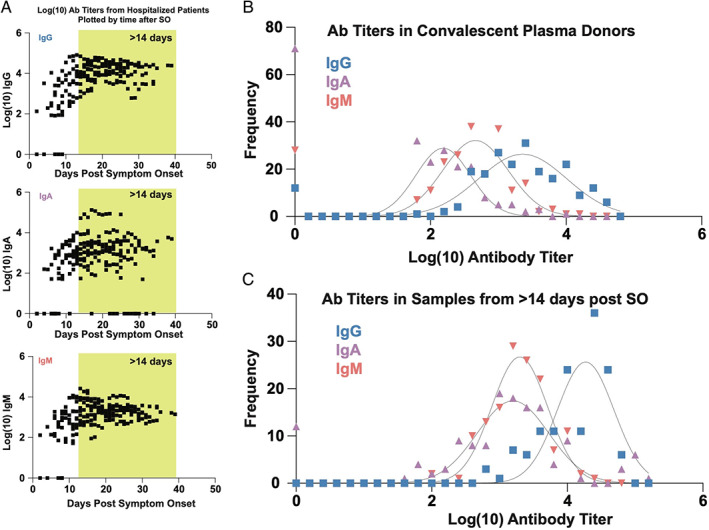
Population level longitudinal analysis of SAR‐CoV‐2 receptor‐binding domain antibodies in hospitalized patients over time. (A) Logarithmic endpoint titers from 201 samples collected from hospitalized PCR‐confirmed COVID‐19 patients are plotted over time after symptom onset. (B, C) Frequency distribution (histogram) analysis of class‐specific SARS‐CoV‐2 endpoint titers in 220 convalescent plasma units (B) and 172 longitudinal samples collected >14 days after symptom onset (shaded yellow in Figure 2(A))
To more fully define the overall abundance of IgA, IgG, and IgM SARS‐CoV‐2 antibody levels in hospitalized patients and CP donors in general, we next compared the relative levels of IgA, IgG, and IgM among CP donors and COVID‐19 hospitalized patients. Despite donating 28 days post‐symptom resolution, IgM antibody levels were sustained in many CP donors, often exceeding corresponding IgA antibody titers (Figure 2(B)). IgA and IgM levels were uniformly lower than corresponding IgG levels in both CP donors and hospitalized patients (Figure 3(B, C)). These results suggest that even following prolonged hospitalization, some patients may not generate robust IgA anti‐SARS‐CoV‐2 antibody responses, at least as detected in plasma. Furthermore, as a large percentage of CP units also possess low IgA levels, transfusion of such IgA poor CP units may not possess the ability to increase IgA levels in patients.
3.4. Decay rates of SARS‐CoV‐2 RBD antibodies in repeat convalescent donors
Differences in IgG, IgM, and IgA levels detected between individual CP donors and hospitalized patients motivated us to next define whether differences may also exist in isotype production over time among repeat CP donors. Examination of IgG, IgM, and IgA levels in CP units from the same individuals (N = 20) at two separate donations demonstrated that while IgG levels were largely sustained, IgM and IgA levels declined more rapidly (Figure 4(A–D)). These results suggest that in addition to distinct immune responses that appear to differentially impact the relative abundance of isotypes in a given CP donor, anti‐SARS‐CoV‐2 antibodies have distinct half‐lives following symptom resolution.
FIGURE 4.

SARS‐CoV‐2 receptor‐binding domain antibodies in repeat convalescent donors. (A–C) Analysis of antibody levels by log endpoint titer over time in repeat convalescent donors (N = 20); repeat donors who did not have detectable antibodies at the outset for a given class were excluded from the % decrease analysis (D). D1, first donation; D2, second donation [Color figure can be viewed at wileyonlinelibrary.com]
3.5. Correlation of Ortho VITROS COVID‐19 antibody test with class‐specific RBD endpoint titers in CP units
While the serological assay we employed is designed to detect isotype‐specific antibody levels, some blood providers utilize the Ortho VITROS test for total anti‐SARS‐CoV‐2 antibody assessment. This assay is designed to examine the presence of SARS‐CoV‐2‐specific antibodies irrespective of isotype. However, whether this test is influenced by a given isotype or may accurately correlate with IgA levels remains incompletely understood. To determine whether this approach provides sufficient information regarding IgA antibody levels in particular, we compared titers with signal to cutoff (S/Co) values reported by the instrument. IgG anti‐SARS‐CoV‐2 antibody titers exhibited the highest correlation, with an R 2 value of 0.52 (Figure 2(E)). In contrast, neither IgA nor IgM antibody levels exhibited a strong S/Co correlation, suggesting that this approach, while capable of assessing the presence or absence of anti‐SARS‐CoV‐2 antibodies, does not possess the ability to accurately assess IgA anti‐SARS‐CoV‐2 antibody levels (Figure 5).
FIGURE 5.
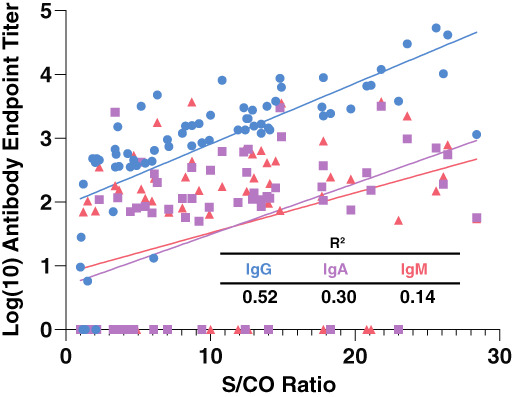
Correlation of Ortho VITROS COVID‐19 antibody test with class‐specific receptor‐binding domain endpoint titers in convalescent plasma units. Linear regression analysis of class‐specific SARS‐CoV‐2 antibody levels compared to the signal to cutoff (S/CO ratio) used to characterize units prior to donation. OV, Ortho VITROS; S/CO, Ortho VITROS signal to cutoff ratio value [Color figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
As the implementation of serological assessment tools lagged behind PCR‐based diagnostic strategies in the early phases of the pandemic, initial attempts to utilize CP as a therapeutic intervention for COVID‐19 understandably relied on PCR confirmed test results in the absence of SARS‐CoV‐2 antibody assessment prior to transfusion. 14 , 15 , 16 This is exemplified by the case presented here, where the antibody levels were not only unknown, but also found to be highly variable in each unit once tested. This variability, particularly with respect to IgA SARS CoV‐2 antibody levels, prompted us to examine a wider number of CP donors, which likewise demonstrated significant differences in both total SARS CoV‐2 antibody levels and individual SARS CoV‐2 isotypes. Importantly, IgG levels did not correlate sufficiently with IgA levels to infer IgA SARS‐CoV‐2 content in a given unit. Thus, while serological tests are now routinely employed in blood donor centers and certainly enhance the practice of CP therapy, most of these platforms do not possess the ability to examine individual SARS‐CoV‐2 isotypes. As IgA is secreted along the respiratory mucosa and little viremia is often detected in patients with COVID‐19, 5 , 6 , 7 , 25 , 26 IgA SARS‐CoV‐2 antibodies represent one variable that may be important when considering optimal approaches to utilizing CP therapy.
In addition to measuring total and isotype‐specific SARS‐CoV‐2 antibody levels in CP units, a similar examination of patients prior to CP therapy may be equally beneficial. The results of the present study suggest that most patients develop high titer IgM, IgG, and IgA levels >14 days after symptom onset, suggesting that a lack of SARS‐CoV‐2 antibodies may be less likely to contribute to ongoing symptoms in patients experiencing a protracted course of COVID‐19. Consistent with this, several studies suggest that patients with prolonged COVID‐19 often possess low or no detectable virus, while exhibiting responsiveness to anti‐inflammatories, such as dexamethasone. 27 These results suggest that the later stages of the disease may more likely reflect a misdirected inflammatory response initiated by SARS‐CoV‐2 than a direct consequence of ongoing uncontrolled SARS‐CoV‐2 infection. CP therapy may therefore be most promising early in the course of the disease, consistent with recent reports outlining the possible benefit of CP in treating COVID‐19. 1 , 2
It is important to note that these studies were not designed to examine the exact role of IgA in the overall efficacy of CP therapy; the optimal SARS‐CoV‐2 titers and overall efficacy of CP therapy in general remain controversial and certainly lie beyond the scope of the present study. Furthermore, whether the lack of IgA SARS CoV‐2 antibody levels in plasma reflect a similar deficiency along the respiratory mucosa in an individual patient remains unknown. More detailed considerations of IgA subclasses and configurations, including levels of IgA1 and IgA2 in addition to the relative concentration of dimeric versus monomeric IgA present in CP units and hospitalized patients may also be important when considering key characteristics of this therapy. The heterogeneity in IgA SARS‐CoV‐2 antibodies in both CP donors and patients, in addition to the more rapid decline of IgA than IgG SARS‐CoV‐2 antibodies following subsequent donations, suggests that unique features of humoral immunity may need to be considered when exploring ways to fully optimize or even determine whether CP is actually effective in treating this disease. Exploring these variables in larger studies may also be especially important when seeking to fully define the risk benefit ratio of this therapy. While early studies suggest that CP therapy may be safe, 15 the long‐term complications that may arise from this therapy, including thromboembolic events that commonly complicate COVID‐19, 28 , 29 , 30 have been more difficult to ascertain. Like delayed type hemolytic transfusion reactions, 31 , 32 , 33 , 34 , 35 , 36 these complications may not be apparent during or even shortly following transfusion and therefore may be more easily missed given the propensity of hospitalized patients with COVID‐19 to experience underlying thromboembolic complications. 28 , 29 , 30 Thus, while intriguing, these observations are correlative in nature and therefore establishing the role of IgA in CP treatment efficacy is not possible with this study. However, our results suggest that IgA antibody levels may be an important consideration when seeking to characterize CP units and patients who may benefit from this approach.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
ACKNOWLEDGMENTS
We appreciate the support of Jeannette Guarner, David Alter, Lori Russell, Jennifer Rha, Heather Lin, Jessica Issac and associated staff for assisting with sample collection and the adjudication of symptom onset following chart review under institutional review board approval (IRB #00022371). We further acknowledge Jens Wrammert and Eric Ortland for providing recombinant RBD for the initial development of our endpoint titer ELISA.
Verkerke H, Saeedi BJ, Boyer D, et al. Are We Forgetting About IgA? A Re‐examination of Coronavirus Disease 2019 Convalescent Plasma. Transfusion. 2021;61:1740–1748. 10.1111/trf.16435
Contributor Information
Cassandra D. Josephson, Email: cjoseph@emory.edu.
Sean R. Stowell, Email: srstowell@bwh.harvard.edu.
REFERENCES
- 1. Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID‐19: initial three‐month experience. medRxiv. 2020. [Google Scholar]
- 2. Libster R, Perez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high‐titer plasma therapy to prevent severe Covid‐19 in older adults. N Engl J Med. 2021;384(7):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katz LM. (A little) clarity on convalescent plasma for Covid‐19. N Engl J Med. 2021;384(7):666–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burton DR, Woof JM. Human antibody effector function. Adv Immunol. 1992;51:1–84. [DOI] [PubMed] [Google Scholar]
- 5. Corthesy B. Multi‐faceted functions of secretory IgA at mucosal surfaces. Front Immunol. 2013;4:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Renegar KB, Small PA Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173:1978–86. [DOI] [PubMed] [Google Scholar]
- 7. Lombana TN, Rajan S, Zorn JA, Mandikian D, Chen EC, Estevez A, et al. Production, characterization, and in vivo half‐life extension of polymeric IgA molecules in mice. MAbs. 2019;11:1122–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leong KW, Ding JL. The unexplored roles of human serum IgA. DNA Cell Biol. 2014;33:823–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansen IS, Baeten DLP, den Dunnen J. The inflammatory function of human IgA. Cell Mol Life Sci. 2019;76:1041–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lecocq M, Detry B, Guisset A, Pilette C. FcalphaRI‐mediated inhibition of IL‐12 production and priming by IFN‐gamma of human monocytes and dendritic cells. J Immunol. 2013;190:2362–71. [DOI] [PubMed] [Google Scholar]
- 11. Rossato E, Ben Mkaddem S, Kanamaru Y, Hurtado‐Nedelec M, Hayem G, Descatoire V, et al. Reversal of arthritis by human monomeric IgA through the receptor‐mediated SH2 domain‐containing phosphatase 1 inhibitory pathway. Arthritis Rheumatol. 2015;67:1766–77. [DOI] [PubMed] [Google Scholar]
- 12. Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID‐19. J Clin Invest. 2020;130:2757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stowell SR, Guarner J. Role of serology in the coronavirus disease 2019 pandemic. Clin Infect Dis. 2020;71:1935–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Joyner MJ, Wright RS, Fairweather D, Senefeld JW, Bruno KA, Klassen SA, et al. Early safety indicators of COVID‐19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130(9):4791–4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;158:e9–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang HE, Ostrosky‐Zeichner L, Katz J, Wanger A, Bai Y, Sridhar S, et al. Screening donors for COVID‐19 convalescent plasma. Transfusion. 2020;61(4):1047–105. [DOI] [PubMed] [Google Scholar]
- 18. Bradfute SB, Hurwitz I, Yingling AV, Ye C, Cheng Q, Noonan TP, et al. Severe acute respiratory syndrome coronavirus 2 neutralizing antibody titers in convalescent plasma and recipients in New Mexico: an open treatment study in patients with coronavirus disease 2019. J Infect Dis. 2020;222:1620–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Suthar MS, Zimmerman M, Kauffman R, Mantus G, Linderman S, Vanderheiden A, et al. Rapid generation of neutralizing antibody responses in COVID‐19 patients. Cell Rep Med. 2020;1(3):100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Verkerke H, Horwath M, Saeedi B, Boyer D, Allen JW, Owens J, et al. Comparison of antibody class specific SARS‐CoV‐2 serology for the diagnosis of acute COVID‐19. J Clin Microbiol. 2021;59(4):e02026–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodriguez ZSA, Verkerke H, Lough C, Zimmeran MG, Suthar M, Wrammert J, et al. COVID‐19 convalescent plasma clears SARS‐CoV‐2 refractory to remdesiivir in an infant with congenital heart disease. Blood Adv. 2020;4(18):4278–4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tait AR, Malviya S, Voepel‐Lewis T, Munro HM, Seiwert M, Pandit UA. Risk factors for perioperative adverse respiratory events in children with upper respiratory tract infections. Anesthesiology. 2001;95:299–306. [DOI] [PubMed] [Google Scholar]
- 23. Malviya S, Voepel‐Lewis T, Siewert M, Pandit UA, Riegger LQ, Tait AR. Risk factors for adverse postoperative outcomes in children presenting for cardiac surgery with upper respiratory tract infections. Anesthesiology. 2003;98:628–32. [DOI] [PubMed] [Google Scholar]
- 24. Mallory MD, Travers C, McCracken CE, Hertzog J, Cravero JP. Upper respiratory infections and airway adverse events in pediatric procedural sedation. Pediatrics. 2017;140(1):e20170009. [DOI] [PubMed] [Google Scholar]
- 25. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chang L, Yan Y, Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev. 2020;34:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Group RC , Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid‐19—preliminary report. N Engl J Med. 2020;384(8):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maier CL, Truong AD, Auld SC, Polly DM, Tanksley CL, Duncan A. COVID‐19‐associated hyperviscosity: a link between inflammation and thrombophilia? Lancet. 2020;395:1758–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Truong AD, Auld SC, Barker NA, Friend S, Wynn AT, Cobb J, et al. Therapeutic plasma exchange for COVID‐19‐associated hyperviscosity. Transfusion. 2021;61(4):1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Narbey D, Habibi A, Chadebech P, Mekontso‐Dessap A, Khellaf M, Lelievre JD, et al. Incidence and predictive score for delayed hemolytic transfusion reaction in adult patients with sickle cell disease. Am J Hematol. 2017;92:1340–8. [DOI] [PubMed] [Google Scholar]
- 32. Yazdanbakhsh K, Ware RE, Noizat‐Pirenne F. Red blood cell alloimmunization in sickle cell disease: pathophysiology, risk factors, and transfusion management. Blood. 2012;120:528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chonat S, Quarmyne MO, Bennett CM, Dean CL, Joiner CH, Fasano RM, et al. Contribution of alternative complement pathway to delayed hemolytic transfusion reaction in sickle cell disease. Haematologica. 2018;103(10):e483–e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chonat S, Arthur CM, Zerra PE, Maier CL, Jajosky RP, Yee MEM, et al. Challenges in preventing and treating hemolytic complications associated with red blood cell transfusion. Transfus Clin Biol. 2019;26:130–4. [DOI] [PubMed] [Google Scholar]
- 35. Chonat S, Graciaa S, Shin HS, Newton JG, Quarmyne MO, Boudreaux J, et al. Eculizumab for complement mediated thrombotic microangiopathy in sickle cell disease. Haematologica. 2020;105(12):2887–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thein SL, Pirenne F, Fasano RM, Habibi A, Bartolucci P, Chonat S, et al. Hemolytic transfusion reactions in sickle cell disease: underappreciated and potentially fatal. Haematologica. 2020;105:539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]


