FIGURE 4.
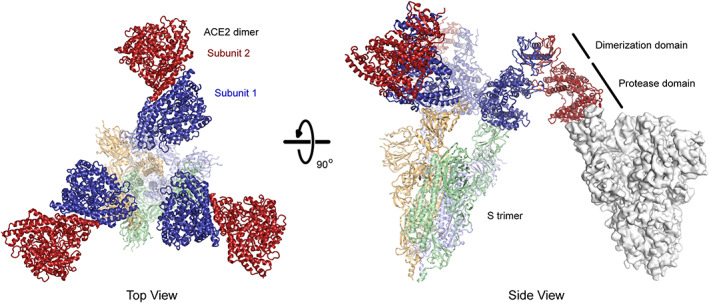
The predicted basis for avid binding of natural ACE22 dimers is inter‐spike bridging. Three copies of ACE22 dimerized via the collectrin‐like domain (subunits in dark red and blue; PDB 6M17) are overlaid with the structure of trimeric SARS‐CoV‐2 S (subunits are pale shades; PDB 7KMS) with all three RBDs in the up conformation and bound to ACE2 protease domains. Note that in each ACE22 dimer, subunit 1 (blue) is bound to a RBD while subunit 2 (red) is directed away from the spike axis where it is accessible for a bridging interaction to another spike (an example is shown as a gray surface at right with a single RBD in the up conformation; PDB 7KNB). Artificial oligomers of sACE2 missing the natural collectrin‐like dimerization domain are not limited to this geometry and might bind with intra‐spike avidity. ACE2, angiotensin‐converting enzyme 2; RBD, receptor‐binding domain; SARS‐CoV‐2, SARS coronavirus 2
