Abstract
The development of a successful vaccine against severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), the agent of coronavirus disease 2019 (COVID‐19), in an unmatched period of ten months, is a tribute to human ingenuity in the face of a vicious pandemic. A return to pre‐pandemic “normalcy” depends on the successful delivery of the vaccine to a majority (~70%) so as to develop herd immunity critical to arrest the community spread of infection. Vaccination against COVID‐19 is particularly important for dentistry as the dental team works in an environment replete with aerosol‐generating procedures (AGP) that facilitate virus spread. Hence, a COVID‐19 vaccine is likely to be an obligatory requirement for the dental practice, and the latest addition to the extensive list of vaccines required for dental professionals for the safe delivery of dental care. Here, we review the currently available major candidate vaccines against SARS‐CoV‐2 and their benefits and risks. These include the vaccines developed on next‐generation platforms (mRNA, DNA, and viral vector vaccines), and the classic platforms (the live‐attenuated virus, and the protein subunit vaccines) The review concludes with a summary of impending issues and challenges facing the provision of COVID‐19 vaccines for all stakeholders in dentistry.
Keywords: challenges, coronavirus disease 2019, impact, platforms, severe acute respiratory syndrome coronavirus 2, vaccines
1. INTRODUCTION
Humankind has survived waves of bacterial and viral plagues over the millennia. The earliest recorded pandemic is considered to be the Babylon flu epidemic around 1200 B.C. Subsequently, there have been many others, including the 14th century European bubonic plague, caused by the bacterium Yersinia pestis, and the influenza pandemic of 1918 known to have killed a third of the then world's population (Norrie, 2016). Although the mortality rate of the current coronavirus disease 2019 (COVID‐19) pandemic is unlikely to approach these figures, it has already killed over 1.8 million people globally (World Health Organization‐report, 2021). The raging, runaway pandemic in all four corners of the world testifies to the persistence, infectivity, and virulence of the causative agent—the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2; Samaranayake, 2020).
Indeed, barely a year after its arrival, we are already seeing more infectious variants of SARS‐CoV‐2 emerging in the UK, South Africa, and Brazil and gaining a firm toehold in many other locales (CDC, 2021b). Their heightened transmissibility, 3–4 times the original counterpart (World Health Organization, 2020a), has been a major concern together with the likelihood that they may cause more severe illness. It is now clear that the societal behavior modifications and the antiviral and other therapeutic measures alone are unlikely to rein in the pandemic. The last great hope for its eradication from the face of the earth is most likely through an effective and efficacious global COVID‐19 vaccination program.
Remarkably, within a few weeks after the beginnings of the then epidemic in Wuhan, Chinese scientists isolated the viral agent of COVID‐19, subsequently named as SARS‐CoV‐2 (by the World Health Organization), and sequenced its ribonucleic acid (RNA) genetic code and shared it with the world (Wang, Li, et al., 2020). This triggered an intense global competition to develop a vaccine against the pandemic virus, with competing vaccine developers using novel technical paradigms and next‐generation vaccine platforms, as well as traditional vaccine technology to accelerate their production and win the “vaccine race”(Logunov et al., 2020; Polack et al., 2020; Ramasamy et al., 2020; Zhu et al., 2020). Although initially there were over 200 vaccine candidates in the research phase, only about 60 have thus far survived the grueling, drawn‐out, and costly vaccine development programs, entailing extensive, triphasic, human trials with thousands of recruits (Dong et al., 2020; Funk et al., 2020; World Health Organization, 2020c). Of the COVID‐19 vaccines currently in contention, only a handful have thus far been approved for emergency use in Europe, UK, USA, as well as in over 20 other jurisdictions (U.S. Food and Drug Administration, 2020b, 2020d; Logunov et al., 2020; Ramasamy et al., 2020). A majority of the latecomer vaccines in the pipeline should see the light of day during this year, once they complete their final (Phase 3) human trials, safety and efficacy proven, and approved by the regulatory authorities (World Health Organization, 2020c).
As mentioned, those developing COVID‐19 vaccines are currently using both traditional as well as novel, next generating vaccine platforms for this purpose (Poland et al., 2020). Up to six major vaccine platforms, and combinations thereof, are currently in vogue, and given below is an overview of the major vaccine platforms and their mechanisms (U.S. Food and Drug Administration, 2020c; Keech et al., 2020; Logunov et al., 2020; Polack et al., 2020; Ramasamy et al., 2020; World Health Organization, 2020c; Zhang et al. 2021; Zhu et al., 2020) (Table 1).
TABLE 1.
Key Advantages and disadvantages, and efficacy of approved major COVID‐19 vaccines (vaccines in late developmental phase are also shown, Classic platforms in Yellow and Next generation platforms in blue); Data form various sources
| Major groups (Classic or Next generation) | Vaccine platform | Advantages | Disadvantages | Vaccines currently approved and claimed efficacy | Vaccines in late developmental phases |
|---|---|---|---|---|---|
| Classic |
Live‐Attenuated Virus |
Relatively easy and quick to manufacture due to proven technology; safe; provides multivalent antigens and hence may protect against current or future viral variants; no adjuvants required; induce strong immune responses | Requires facilities for live virus handling; inappropriate for immunosuppressed individuals; Rare possibility of reversion and risk for infection; complicated to scale up manufacturing | Nil in the market thus far | Covi‐Vac intranasal vaccine(Codagenix/Serum Institute of India) |
| Classic | Inactivated virus | Relatively easy and rapid manufacture due to proven technology; provides multivalent antigens and hence may protect against current or future viral variants; no adjuvants required | Complex manufacturing process and hence difficulty to scale up; less reactive (reactogenic) than the live virus vaccines and produces a weaker immune response | Sinovac Vaccine(Sinovac Research and Development Co., Ltd./Butantan Institute) (Efficacy: 52 percent) | QazCovid (Research Institute for Biological Safety Problems, India) |
| Sinopharm vaccine(Sinopharm Group, China)(Efficacy: 79 percent) | |||||
| Classic | Protein/Subunit | Relatively safe as live virus is not used in the actual vaccine manufacture; induces strong cellular and humoral immune responses | High cost; lower immunogenicity to specific proteins/antigens; adjuvants required to increase potency may cause allergic reactions in vaccinees; complicated to scale up the manufacturing process | Novavax, Inc. (Nasdaq: NVAX) NVX‐CoV2373 COVID−19 vaccine(Efficacy: 89.3% in the United Kingdom and 49.4% in South Africa—including HIV‐positive and HIV‐negative subjects) | NVX‐CoV2373 (Novavax, USA).SCB−2019 vaccine (Clover Biopharmaceuticals AUS Pty Ltd.), Covax−19 (GeneCure Biotechnologies; Vaxine Pty Ltd.), |
| Next generation | Viral Vector‐Based | High degree of safety due to years of proven experience in the gene therapy field; induces strong cellular and humoral responses as they imitate the natural infection; can be stored at 40C (home fridge temperature) | Theoretical risk of infection and/or chromosomal integration and oncogenesis; ineffective in immunocompromised subjects (as most have been pre‐exposed to multiple adenoviruses) | Oxford–AstraZeneca COVID−19 vaccine (AZD1222); AstraZeneca and University of Oxford, UK (Efficacy: 72 percent) | CanSino Biologics. |
| Gamaleya (Sputnik V) Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation.(Efficacy: 92 percent) | |||||
| Johnson & Johnson; USA(Efficacy: 66–85 percent) | |||||
| Next generation | mRNA | Safe as no live virus handling during development, rapid development, and production; flexibility to produce multivalent vaccines (in the event of virus variants arising); no risk of genetic integration; induces a very strong immune response | RNA being very fragile require ultra‐cold chain requirements (−700C for Moderna and −20 0 C for Pfizer/Biotec) for storage and transport; currently, high cost; long term adverse effects, if any, unknown |
Moderna, Moderna, USA (Efficacy: 95 percent) |
CureVac (Bayer and Imperial College London) |
|
Pfizer‐BioNTec :Europe (synonym: Cominarty) In adults (Efficacy: approx. 95 percent) In children, aged 12–15‐years (Efficacy: 100 percent) | |||||
| Next generation | DNA | Safe as no live virus handling during development; possibility of multivalency; both humoral and cell‐mediated immune response, good long‐term stability with no cold chain requirements; possibility of freeze drying and storage and transportation at ambient temperatures; oral formulations are feasible | Repeated doses thought to cause toxicity; potential risk of genetic integration unknown | Nil in the market thus far |
INO−4800 (International Vaccine Institute; Inovio Pharmaceuticals), Symvivo, Canada ‐COVID19 (AnGes, Inc.); GX−19 (Genexine, Inc.) |
Introducing a new vaccine into the global community is an opportunity to curb the rabid infection and a long‐awaited prospect to return to the pre‐COVID‐19 “normalcy” of delivering dental care. However, vaccinating a global population is weighed down by a staggering number of issues ranging from supply‐chain logistics to myths and fears on vaccine efficacy and side effects leading to so‐called “vaccine hesitancy” (syn: anti‐vaccination, anti‐vax) by a significant proportion of the populace (World Health Organization, 2017, 2020b). Similarly, the dental team will have to contend with its own share of challenges related to the introduction of a new vaccine developed on hitherto untested vaccine platforms, and the so‐called vaccine complacency.
In the final section of this overview, we examine these looming concerns and logistical issues facing both the profession and the public. Nevertheless, first, a rejoinder on the general mechanisms underlying vaccine immunology, followed by a short description of the five major COVID‐19 vaccine platforms.
2. VACCINE IMMUNOLOGY: THE BASICS
In brief, a vaccine aims to stimulate the body's own protective immune response, mainly antibody‐producing B cells, and the enabling T cells (such as cytotoxic T cells) so that, in the event of a future encounter with the specific viral pathogen, then the immune system can quickly recognize the virus, jump into action, kill the invading viral army, prevent the spread of infection, and abort the disease (Samaranayake, 2018). In the case of vaccines for SARS‐CoV‐2, the goal is to produce antibodies against the spike (S) protein or the proteins on the receptor‐binding domain (RBD) of the spikes (Du et al., 2009; Speiser & Bachmann, 2020; Figure 1).
FIGURE 1.
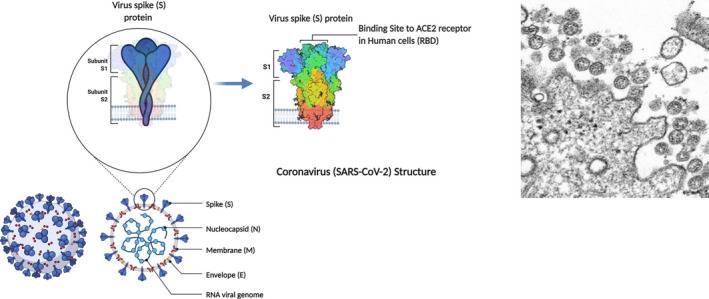
SARS‐CoV‐2 and its structural components: Showing the (blown up) spike (S) protein and receptor‐binding domain (RBD) that are key antigenic components of COVID‐19 vaccines; also illustrated are the N (nucleocapsid), M (membrane), and S (spike) proteins of the virus, which are the key components of the subunit vaccines; Inset: Transmission electron microscope image of SARS‐CoV‐2: spherical viral particles emerging from an infected cell (Image courtesy US Centres for Disease Control Image Library)
It is now well established that the numerous spikes on the viral surface initiate its attachment to the susceptible human cells (via the receptor binding proteins), allowing it to enter the cell and hijack the DNA of the cell, to produce a progeny that will re‐infect other cells and spread the infection. Hence, neutralizing the critical functionality of the protruding viral spikes that facilitates viral entry, with the vaccine‐induced, preformed antibodies, abetted by the enabling T cells, terminates the infection in its tracks (Du et al., 2009; Grigoryan & Pulendran, 2020). However, over a prolonged post‐vaccination period, the virus‐neutralizing antibody level is likely to wane to almost undetectable levels. This is when the vaccine‐induced "memory" T‐lymphocytes, as well as B‐lymphocytes, come into action during a second and subsequent encounter with either the identical virus or its variant progeny (Speiser & Bachmann, 2020; Figure 2).
FIGURE 2.

A composite figure depicting the mechanisms of activity of five different strains of COVID‐19 vaccines (simplified). The central square delineates the systemic immune response evoked by the spike (S) antigens of SARS‐CoV‐2 produced on various vaccine platforms. (a) Specialized antigen‐presenting cells (APCs: dendritic cells, macrophages, B cells and Langerhans cells (Purple) engulf the S protein antigens produced on different vaccine platforms (described below) and display portions of it on their cell surfaces to activate and recruit T cells; (b) The recruited T cells, so‐called killer T cells (red), then destroy SARS‐CoV‐2‐infected cells; (c) APC also recruit B cells (green) that are primed to produce neutralizing antibodies to the viral S proteins, preventing viral attachment to host cells and abating the infection. (d) Long‐lived memory B and T cells (light brown) are also produced simultaneously that can patrol the body for any incoming viruses for months/years and rekindle an identical B‐ and T‐cell response, described above. I: mRNA Vaccines; The vaccine containing mRNA of SARS‐CoV‐2 with the code for the Spike (S) protein enclosed within lipid particles (to facilitate cell entry) is administered. Once injected, the lipid particles are “ingested” by vaccinees APCs to produce the immune response (as described in (a‐d) above). IIa and IIb: Live attenuated and inactivated vaccines; The vaccine containing either the inactivated or the attenuated SARS‐CoV‐2 is administered to the vaccinee. Once injected, the non‐infective virus particles are ingested by the antigen‐presenting cells (APC ) to produce the immune response (as described in (a‐d) above). III: Protein subunit SARS‐CoV‐2 vaccines: The vaccine containing the N (nucleocapsid), M (membrane), and S (spike) protein antigens of the virus is administered to the vaccinee. Once injected, the non‐infective antigens are ingested by the antigen‐presenting cells (APC) (purple) to produce the immune response (as described in (a‐d) above). IV: Viral vector‐based recombinant vaccines: A non‐infectious virus such as an adenovirus, engineered to combine with SARS‐CoV‐2 Spike (S) protein (recombinant) DNA is used as a vector vehicle in the vaccine. Once injected, the non‐infective adenoviruses multiply in host cells, recognised as foreign, and ingested by the antigen‐presenting cells (APC; purple) to produce the immune response (as described in (a‐d) above); V: DNA vaccines: These vaccines contain pieces of DNA called plasmids found in bacteria to encode spike (S) antigens of SARS‐CoV‐2. The plasmids with a code to produce S antigens, once ingested by APC are integrated into their nucleus (purple) to produce the immune response (as described in (a‐d) above; Figure produced using Biorender.com)
2.1. Clinical implications of vaccine‐induced immunity
Antibodies produced by the B cells of the immune system through a vaccination process can vary both in quantity as well as in quality. In general, antibodies can be divided into two basic types: neutralizing (NAb) and non‐neutralizing antibodies (nNAb). The antibodies that block entry of the pathogen into the host cells, and stop the infection, termed neutralizing antibodies, should be distinguished from the binding antibodies or non‐neutralizing antibodies (nNAB). The latter merely binds to the pathogen but does not necessarily interfere with viral entry into the host cells, possibly because they do not interact with the correct structural anatomic region of the virus to disable it. However, binding antibodies play a contributory “accessory role” by signaling the immune cells of the impending viral invasion, after which the virus is processed and destroyed by the recruited immune cells (Schmaljohn, 2013).
The neutralizing antibodies (NABs), on the other hand, can neutralize the virus single‐handedly, as it were without the assistance of other immune cells. For instance, in the case of COVID‐19, the neutralizing antibodies bind to the spike (S) antigens on the viral surface and render the “spikes” ineffective in attaching to the host cells, as mentioned above (Figure 2). The currently approved COVID‐19 vaccines are all extremely effective in producing these neutralizing antibodies (U.S. Food and Drug Administration, 2020a, 2020c, 2021; Ramasamy et al., 2020).
The neutralizing antibody response of an individual can be further sub‐divided into two different types, called the effective immunity, and the sterilizing immunity (Kumar et al., 2018). There is a significant clinical difference between these slightly divergent immune responses of a vaccinee. Thus, in the case of effective immunity the antibody response prevents vaccinee from contracting the illness, yet he/she may become an asymptomatic silent carrier of infection over a period of time. Consequently, the vaccinee may unknowingly become a “silent spreader” of the infection for an indeterminate period. On the contrary, in the case of sterilizing immunity, the very high level of seroconversion totally aborts the virus multiplication in vaccinees cells and prevents the further transmission of the virus to another (Kim et al., 2020). Not surprisingly, therefore, sterilizing immunity is the desired goal of all vaccine manufacturers.
It is still unclear which of the COVID‐19 vaccines available provide which type of immunity, to what extent, and in what proportion of the vaccinees, until all the clinical trials are completed, and mass vaccination results are evaluated. Nonetheless, irrespective of the type of bi‐pronged immunity conferred by the current COVID‐19 vaccines, there is still hope, as there are a number of vaccine precedents for epidemic‐prone diseases, such as measles, polio, and hepatitis B, where the vaccination process does not produce sterilizing immunity, and yet the spread of community infection has been successfully curtailed (Samaranayake & Anil, 2021).
Finally, in this context, it is also noteworthy that the `artificial` vaccine‐induced “infection” may cause minor symptoms in the vaccinee immediately after vaccination. These, such as soreness of the injection site and mild fever (Kohl et al., 2004), are rarely as severe as the natural infection, which may cause life‐threatening disease. Second, as it takes approximately 4–5 weeks for the priming and activation of the B and T lymphocytes and immunity to be established, a vaccinee is not fully protected until then. Hence, there are situations where the vaccinees may contract COVID‐19 immediately before or after vaccination (as has been reported recently), an outcome that should not be attributed to vaccine failure (CDC, 2021a).
3. COVID‐19 VACCINES AND VACCINE PLATFORMS
At the time of writing (April 4, 2021), there were 23 vaccines in large scale clinical trials (Phase III), six vaccines in early or limited use and seven vaccines approved for full use (CDC, 2021a). The next section outlines the principles, advantages, and disadvantages of the five major vaccine platforms, details of minor groups such as re‐purposed BCG vaccines are not provided here for the sake of clarity. First, the three major classic vaccine platforms, live attenuated virus, whole inactivated virus, and virus‐like particle/subunit vaccines are described, followed by the next generation platform vaccines mRNA, DNA, and viral vector vaccines (Tables 1 and 2, Figure 2).
TABLE 2.
Manufacturing platforms, immunization attributes and examples of different COVID‐19 vaccines (either currently available or in developmental pipeline; minor platforms are not shown)
| Text reference(technology) | Vaccine Platform | Illustrative figure | Mode of Action | Examples of SARS‐CoV−2 vaccine (currently available, and developmental) | Immunization attributes* | Currently available for these infections (not an exhaustive list) | Viral Vaccines currently prescribed for dental care workers |
|---|---|---|---|---|---|---|---|
| I and II (Classical) | Inactivated or attenuated virus |
(See also Figure 2)
|
Vaccines created from weakened SARS‐CoV−2 or those attenuated with chemicals |
Inactivated: Sinovac Biotech, Sinopharm, the Wuhan Institute of Biological Products, and Bharat Biotech (India) Attenuated: Covi‐Vac, Codagenix/Serum Institute of India: Indian Immunologicals Ltd/Griffith University |
Expresses multiple viral antigens. | Inactivated : Hepatitis A, Polio (IPO, Salk variant): Attenuated: Measles, Mumps, and Rubella (MMR), Varicella, Influenza, Whooping cough (pertussis); Oral Polio Vaccine(Sabin variant) | Measles, Mumps, and Rubella (MMR), Varicella, Influenza |
| III (Classical) | Protein based /sub‐unit |
(See also Figure 2)
|
Vaccines that contain SARS‐CoV−2 proteins only, either whole protein, or fragmented, sub‐units. Some pack many of these molecules in nanoparticles |
NVX‐CoV2373 (Novavax, USA). SCB−2019 vaccine (Clover Biopharmaceuticals AUS Pty Ltd.), Covax−19 (GeneCure Biotechnologies; Vaxine Pty Ltd.), |
Recombinant Spike (S) or receptor binding domain (RBD) proteins | Hepatitis B, acellular Whooping cough (Pertussis) | Hepatitis B |
| IV (Next generation) | Viral Vector (replicating/non‐replicating) |
(See also Figure 2)
|
Viruses engineered to carry coronavirus genes (Trojan horse principle), but non‐replicating, enter receptive cells and instruct them to make viral proteins or slowly replicate, carrying coronavirus proteins on their surface. (vector examples: chimpanzee adenovirus, Vaccinia virus) | Oxford‐AstraZeneca, Johnson & Johnson, CanSino Biologics, and Gamaleya Research Institute, Health Ministry of the Russian Federation | Expresses S (spike) protein | COVID−19 (AstraZeneca now approved in a number of countries); Ebola infections | Nil |
| V (Next generation) | mRNA Vaccines |
(See also Figure 2)
|
Delivers one or more of SARS‐CoV−2 RNA genes into cells to provoke an immune response | Moderna, Pfizer/BioNTec, CureVac, and Imperial College London. | Expresses S (spike) protein | COVID−19 vaccines Moderna and Pfizer/BionTech (approved in a number of countries) | Nil |
| VI (Next generation) | DNA Vaccines |
(See also Figure 2)
|
Delivers SARS‐CoV−2 DNA genes into cells, with the help of a plasmid (“a jumping gene” found in bacteria) to provoke an immune response | INO−4800 (International Vaccine Institute; Inovio Pharmaceuticals), Symvivo, Canada ‐COVID19 (AnGes, Inc.); GX−19 (Genexine, Inc.) | Expresses S (spike) protein | Veterinary infections (None so far approved for COVID−19) | Nil |
The last column shows the categorization of viral vaccines currently prescribed for dental healthcare workers. (Data from various sources; Adapted from Samaranayake and Fakhruddin, 2021.
All vaccines essentially need two repeated doses to achieve optimal seroconversion and are injected intramuscularly, usually the arm.
3.1. Live‐attenuated vaccines (classic platforms; Tables 1 and 2, and Figure 2)
Examples: (in developmental phase) Covi‐Vac intranasal vaccine, Codagenix/Serum Institute of India, and Indian Immunologicals Ltd/Griffith University (World Health Organization, 2020c)
These vaccine platforms are decades old, and the vaccines essentially contain multiple antigenic components derived from the attenuated live viruses, which are immunogenic but not infectious (Zhang et al., 2020). The advantages include proven technology and long‐lasting and robust immunogenicity, including stimulation of toll‐like receptors. A major drawback of this platform is the need to handle live viruses during manufacture (Wang et al., 2020) and the possibility of adverse reactions in immunocompromised individuals. At present, there are no live‐attenuated COVID‐19 vaccines that have been approved after completing clinical trials.
Good examples of such vaccines currently in use are those against measles, mumps, rubella (MMR), varicella, oral polio (Sabin variant), and intranasal influenza vaccines. MMR and influenzas vaccines are currently recommended for health care workers including dental care workers, Tables 1 and 2.
3.2. Inactivated vaccines (classic platforms; Tables 1 and 2, and Figure 2)
Examples: Sinovac Biotech (Sinovac Research and Development Co., Ltd./ Butantan Institute); Sinopharm; Bharath Biotech.
In comparison with the live attenuated virus vaccines discussed above, these vaccines contain SARS‐CoV‐2, which is inactivated, usually by chemicals so that the antigenicity of the viral surface components are retained. The technology used in this platform is also decades old and proven. Due to the presence of the whole virus with a multiplicity of surface antigenic components, these vaccines induce a diverse immunologic response (Zhang et al., 2020). Nevertheless, they are less reactogenic and produce a weaker immune response than live attenuated vaccines. The manufacturing process of both the live attenuated and inactivated vaccine preparations entail handling live virus preparations, which adds to their complexity. This contrasts with other vaccine platforms described below that use viral components, as opposed to the whole viruses, as the active ingredient of the vaccine (Wang, Li, et al., 2020). Examples of inactivated viral vaccines currently in use are inactivated poliovirus vaccine (IPV or Salk variant) and the hepatitis A vaccine (Sanders et al., 2014), Table 2.
3.3. Protein/subunit vaccines (Classic Platforms; Tables 1 and 2, and Figure 2).
Examples: NVX‐CoV2373: (Novavax); SCB‐2019 vaccine (Clover Biopharmaceuticals AUS Pty Ltd.); Covax‐19 (GeneCure Biotechnologies; Vaxine Pty Ltd.),
The active ingredients of subunit vaccines are the immunogenic proteins, so‐called immune‐protective antigens, of SARS‐CoV‐2 (Table 2), mainly the S or RBD proteins that act as antigens once injected into a vaccinee, (Figure 1). As live virus handling is not required, subunit vaccine manufacturing process is safer and simpler (Romero‐Maldonado et al., 2014). However, one disadvantage is they often require effective adjuvants to obtain a more robust immune response, and on occasions, the adjuvants may cause strong allergic reactions in vaccinees (Zhang et al., 2020).
3.4. Viral vector‐based vaccines (next‐generation platforms) (Tables 1 and 2, and Figure 2)
Example: Oxford–AstraZeneca: COVID‐19 vaccine (AZD1222); AstraZeneca and University of Oxford; Johnson & Johnson; Gamaleya (Sputnik V) Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation. (Others in pipeline: CanSino Biologics; the Gam‐COVID‐Vac (Gamaleya Research Institute of Epidemiology and Microbiology, Health Ministry of the Russian Federation)).
As the name implies, these are based on live recombinant virus vectors to deliver SARS‐CoV‐2 genes/ antigens to the target host tissues (Figure 2). The vector is a viable, though harmless, non‐replicating `carrier' virus that imitates infection caused by SARS‐CoV‐2 and is thought to induce a more robust cellular immune response than the recombinant protein vaccines (Wang, Li, et al., 2020).
Adenovirus, retroviruses, and vaccinia viruses are traditionally used as carrier vehicles in viral vector vaccines (Rauch et al., 2018). For example, in the case of the newly approved Oxford‐AstraZeneca vaccine, the vector virus used is a modified version of a chimpanzee adenovirus, known as ChAdOx1 which can enter human cells but cannot replicate within them (Ramasamy et al., 2020),
Viral vector vaccine platform technology is not new and have been used for many years in the field of gene therapy. The proven experience so garnered is an advantage over the newer platforms such as mRNA vaccines described below. They are also safe as weak virus vectors, due to gene deletion, do not replicate within the cells once injected, leading to a slim, if any, risk of chromosomal integration (Table 2). One drawback of such vaccines, in general, is that they may not be suitable for immunocompromised subjects, as most of them are likely to have been previously exposed to adenoviruses and may have pre‐existing immunity to them (Gray & Erdman, 2018) Such immunity, including the presence of antibodies against adenoviruses, may impedes the entry of the “vaccine strain” of the adenovirus, (i.e., the active component of the vaccine) into the vaccinees/host cells thereby minimizing or aborting the vaccine efficacy (Gray & Erdman, 2018). One way to circumvent this is to utilize animal adenovirus vectors, which can gain entry into human cells but are not human pathogenic. Hence, the Oxford‐AstraZeneca COVID‐19 vaccine utilizes a chimpanzee adenovirus as a vector (Ramasamy et al., 2020).
3.5. Messenger RNA (MRNA) vaccines (next‐generation platforms) (Tables 1 and 2, and Figure 2)
Examples: Moderna, Pfizer‐BioNTec (Others in the pipeline: CureVac/Bayer and Imperial College London).
This is one of the newest yet simplest methods available to insert specific viral genes, such as the spike (S) protein gene of SARS‐CoV‐2, to human cells so as to produce antibodies against them (Figure 2. This technology entails artificial synthesis of messenger RNA of the virus in the laboratory (using a known RNA sequence to produce S or RBD proteins) and incorporating these within lipid nanoparticles (LNP) to protect the fragile RNA as well as to increase the `transfection efficacy.' The lipid nanoparticles act as the transportation vehicle for the surreptitious transfer of the RNA into the immune cells (Schlake et al., 2012). The lipid nanoparticles protect the very fragile RNA strands and prevent their destruction until they reach the active site in the receptor cells (Zhang et al., 2019),
Once injected, as a vaccine, the lipid droplets are “ingested” by vaccinees immune cells. The oily/lipid coat is gradually destroyed, exposing mRNA, after which the protein‐making machinery of the cell is then instructed to produce viral proteins (i.e., S or RBD antigens), which are either presented on the cell surface or released into the bloodstream. When the B or T cells encounter these so‐called “antigen‐presenting cells” with S or RBD antigens, they produce antibodies while simultaneously activating the killer T cells (Jackson et al., 2020; Polack et al., 2020; Sahin et al., 2020). The immune system is now forearmed to attack the invading virus, as it already has antibodies and the T cells to kill the invader (Figure 2).
The significant advantages of mRNA vaccines are their safety, rapidity, and flexibility of design and production (Pardi et al., 2018). As the production process does not entail using either live or attenuated viral vectors, these vaccines are simple to develop and manufacture. Moreover, the strong and quick humoral and cell‐mediated antiviral responses (over 90%) are truly spectacular, compared with vaccines, such as the seasonal flu vaccines with approximately 50%–60% efficacy (Zhang et al., 2019). Finally, as mRNA is not inserted into the (DNA) nuclear material of the immune cells, but slowly destroyed in the cellular cytoplasmic vesicles, there is no likelihood for insertional mutagenesis (Jackson et al., 2020; Sahin et al., 2020).
Despite the above advantages, the biggest drawback of these vaccines is their fragility. The formulations require ultra‐cold chain requirements (−700C) for longevity and stability due to RNA that is easily destroyed (U.S. Food and Drug Administration, 2020a). Hence, the widespread deployment of these vaccines in resource‐poor jurisdictions is highly questionable, as DNA vaccines are far superior for such regions. (For, DNA is highly stable and may be extracted even from remnants of prehistoric animals). Finally, in this context, mRNA vaccines have only been used previously for cancer therapy and not to prevent infections (Pardi et al., 2018). Hence, the long‐term adverse effects of viral RNA vaccines, if any, are not known as yet.
Some of the side effects seen in mRNA vaccines were identical to those seen with traditional vaccines. They included pain and inflammation at the injection site, fatigue, chills, headache, and myalgia. However, some have reported systemic adverse effects that were more severe and frequent after the second vaccination (Jackson et al., 2020).
3.6. DNA vaccines (next‐generation platforms) (TABLES 1, 2, and Figure 2)
Examples: undergoing Phase 2/3 trials including INO‐4800 (International Vaccine Institute; Inovio Pharmaceuticals), Symvivo, Canada ‐COVID19 (AnGes, Inc.); GX‐19 (Genexine, Inc.)
These vaccines use pieces of DNA called plasmids found in bacteria to encode spike (S) antigens of SARS‐CoV‐2. Plasmids are easily transmissible DNA pieces and, as such, called “jumping genes.” In order to produce the S or RBD proteins, these plasmids need to enter (jump into) the nucleus of the antibody‐producing B cells, and plasmids have the intrinsic ability to do so (Liu, 2019, Figure 2).
The significant advantages of this genre of DNA vaccines include, not necessitating live virus handling during preparation, and the possibility of freeze‐drying and long‐term storage and transportation at ambient temperatures (as opposed to extremely fragile mRNA vaccines above) (Wang, Li, et al., 2020). None of the plasmids derived DNA vaccines have been released for general use as yet, although some are in final, phase 3 trials. Currently, DNA vaccines are approved only for use in veterinary practice (Table 2).
The foregoing describes the different major vaccine platforms and COVID‐19 vaccines belonging to these, either currently approved or in various developmental phases. At the time of writing (Feb. 1, 2021), at least ten vaccines were approved for emergency or limited use, and further 20 vaccines in large scale, Phase III, final trials (Zimmer et al., 2021). Once the data on vaccine efficacy from these trials are released, vetted and approved, by various regulatory authorities, it is highly likely that the vast majority of these new vaccines will be released for general administration before the end of 2021. It is likely that there will be a glut of COVID‐19 vaccines of various descriptions, by the end of 2021 and the bottle necks in vaccinating the whole world population are likely to be the delivery and supply chain logistics to remote regions of the world, and the vaccine hesitancy of a substantial proportion of the population, as described in the next section.
The final section of the review addresses salient key issues appertaining to COVID‐19 vaccines, vaccinations, and dentistry, with concluding perspectives.
4. COVID‐19 VACCINES and DENTISTRY
A primary duty and a major obligation of a dental professional is to deliver care in a healthy and safe environment for the patients and the attending co‐workers. A core component of such risk mitigation is to prevent the spread of infectious hazards within the dental clinic by implementing standard infection control measures in dentistry (CDC, 2020; Clementini et al., 2020; Izzetti et al., 2020; Li et al., 2020; Samaranayake, 2018; Volgenant et al., 2020), and enshrined therein is a major stipulation for immunization of all clinical practice personnel against a range of readily transmissible infections. Accordingly, the staff needs to be protected from a number of bacterial and viral infections, through a regimented vaccination procedure, as a buffer against occupationally acquired infections such as hepatitis B and seasonal influenza (World Health Organization, 2015; Table 2; last column). It is highly likely that the COVID‐19 vaccine will now be the newest addition to this extensive list of vaccines obligatory for dental care workers to conduct safe dental practice.
In this context, universities and similar establishments that deliver dental education for undergraduates and postgraduates need to consider how best to manage COVID‐19 vaccination requirements and develop necessary guidelines, as in the case of the current hepatitis B vaccination regimen stipulated for such teaching institutions. In future, all students may be required to produce COVID‐19 immune sufficiency certificates (either electronic or hard copy) at periodic intervals prior to attending college, depending on factors that are yet to be determined. These obligatory requirements should be applied across the board to all academic staff and other clinical or non‐clinical support personnel, as necessary for the benefit of all stake holders including the patients attending such establishments (Khalifa et al., 2021).
5. PERSPECTIVES
We are in the first phase of the COVID‐19 vaccination program, and it is unclear to what extent the foregoing scenarios will transpire in the future. There are still many questions than answers on COVID‐19 vaccines, both related to vaccines in general and dentistry specifically, such as the following: How long will vaccine immunity last and the necessity of periodic booster vaccines, especially for the dental team? What are the correlates of protective immunity following vaccination? Does clinical dentistry increase the risk for infection to patients and the dental staff? Whether the dental/oral pathology visits, which may have been repeatedly delayed or postponed due to the pandemic, impact patients’ outcomes due to late screening, and diagnosis (e.g., oral cancer and other progressive oral diseases), and finally, in view of the lasting impact of the pandemic, the renewed and urgent requirement for developing oral telemedicine.
It will take a few years before we have answers to all these questions. Until then, as responsible health care providers, the dental community has a duty and an obligation to keep abreast of the dynamic developments in the COVID‐19 vaccine ecosystem, particularly the adverse and beneficial effects across the spectrum of vaccines delivered. However, it is crucial for the community to comprehend that COVID‐19 vaccines are but a single arm in the overall public health response to the pandemic, and also the critical necessity to maintain other public health preventive measures such as wearing of masks, social distancing, and maintaining scrupulous hand and personal hygiene (CDC, 2021c). Vaccines are termed the tugboats of preventive health, and in the case of the COVID‐19 pandemic, the currently available raft of vaccines and the newer, impending arrivals in the final stages of approval appear to be the raft of tugboats that will drag the humanity out of this perfect, viral storm.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Lakshman Samaranayake: Conceptualization; Writing‐review & editing. Chaminda Jayampath Seneviratne: Writing‐review & editing. Kausar Sadia Fakhruddin: Writing‐review & editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/odi.13916.
Samaranayake LP, Seneviratne CJ, Fakhruddin KS. Coronavirus disease 2019 (COVID‐19) vaccines: A concise review. Oral Dis. 2021;00:1–11. 10.1111/odi.13916
Samaranayake and Fakhruddin: Co‐corresponding authors.
Contributor Information
Lakshman Perera Samaranayake, Email: lakshman@hku.hk.
Kausar Sadia Fakhruddin, Email: kfakhruddin@sharjah.ac.ae.
REFERENCES
- CDC (2020). Summary of infection prevention practices in dental settings. Centers for Disease Control and Prevention (CDC), https://www.cdc.gov/oralhealth/infectioncontrol/pdf/safe‐care2.pdf, 1‐44. [Google Scholar]
- CDC (2021a). About COVID‐19 Vaccination. Centers for Disease Control and Prevention. https://www.CDC.gov/coronavirus/2019‐ncov/vaccines/faq.html. 1‐3 [Google Scholar]
- CDC . (2021b). Emerging SARS‐CoV‐2 Variants. Centers for Disease Control and Prevention (CDC). https://www.CDC.gov/coronavirus/2019‐ncov/more/science‐and‐research/scientific‐brief‐emerging‐variants.html. 1‐3 [PubMed] [Google Scholar]
- CDC . (2021c). How to protect yourself and others. Centers for Disease Control and Prevention, March 8 2021. 1‐2. [Google Scholar]
- Clementini, M. , Raspini, M. , Barbato, L. , Bernardelli, F. , Braga, G. , Di Gioia, C. , Littarru, C. , Oreglia, F. , Brambilla, E. , Iavicoli, I. , Pinchi, V. , Landi, L. , Marco Sforza, N. , Cavalcanti, R. , Crea, A. , & Cairo, F. . (2020). Aerosol transmission for SARS‐CoV‐2 in the dental practice. A review by SIdP Covid‐19 task‐force. Oral Diseases. 10.1111/odi.13649 [DOI] [PubMed] [Google Scholar]
- Dong, Y. , Dai, T. , Wei, Y. , Zhang, L. , Zheng, M. , & Zhou, F. (2020). A systematic review of SARS‐CoV‐2 vaccine candidates. Signal Transduction and Targeted Therapy, 5(1), 237. 10.1038/s41392-020-00352-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, L. , He, Y. , Zhou, Y. , Liu, S. , Zheng, B.‐J. , & Jiang, S. (2009). The spike protein of SARS‐CoV—a target for vaccine and therapeutic development. Nature Reviews Microbiology, 7(3), 226–236. 10.1038/nrmicro2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk, C. D. , Laferrière, C. , & Ardakani, A. (2020). A Snapshot of the global race for vaccines targeting SARS‐CoV‐2 and the COVID‐19 pandemic. Frontiers in Pharmacology, 11, 937. 10.3389/fphar.2020.00937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, G. C. , & Erdman, D. D. (2018). Adenovirus vaccines. Plotkin's Vaccines, 121–133, e128. 10.1016/B978-0-323-35761-6.00010-9 [DOI] [Google Scholar]
- Grigoryan, L. , & Pulendran, B. (2020). The immunology of SARS‐CoV‐2 infections and vaccines. Seminars in Immunology, 50, 101422. 10.1016/j.smim.2020.101422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzetti, R. , Gennai, S. , Nisi, M. , Barone, A. , Giuca, M. R. , Gabriele, M. , & Graziani, F. (2020). A perspective on dental activity during COVID‐19: The Italian survey. Oral Diseases. 27(S3), 694–702. 10.1111/odi.13606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, L. A. , Anderson, E. J. , Rouphael, N. G. , Roberts, P. C. , Makhene, M. , Coler, R. N. , McCullough, M. P. , Chappell, J. D. , Denison, M. R. , Stevens, L. J. , Pruijssers, A. J. , McDermott, A. , Flach, B. , Doria‐Rose, N. A. , Corbett, K. S. , Morabito, K. M. , O’Dell, S. , Schmidt, S. D. , Swanson, P. A. , … Beigel, J. H. (2020). An mRNA vaccine against SARS‐CoV‐2 — preliminary report. New England Journal of Medicine, 383(20), 1920–1931. 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keech, C. , Albert, G. , Cho, I. , Robertson, A. , Reed, P. , Neal, S. , Plested, J. S. , Zhu, M. , Cloney‐Clark, S. , Zhou, H. , Smith, G. , Patel, N. , Frieman, M. B. , Haupt, R. E. , Logue, J. , McGrath, M. , Weston, S. , Piedra, P. A. , Desai, C. , … Glenn, G. M. (2020). Phase 1–2 Trial of a SARS‐CoV‐2 recombinant spike protein nanoparticle vaccine. New England Journal of Medicine, 383(24), 2320–2332. 10.1056/NEJMoa2026920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalifa, N. , Samaranayake, L. P. , & Fakhruddin, K. S. (2021). Dental pedagogy in the new normal COVID‐19 Era: A transition template of teaching protocols. MedRxiv Preprint, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. S. , Rowland‐Jones, S. , & Gea‐Mallorquí, E. (2020). Will SARS‐CoV‐2 infection elicit long‐lasting protective or sterilising immunity? Implications for vaccine strategies (2020). Frontiers in Immunology, 11(3190), 571481. 10.3389/fimmu.2020.571481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl, K. S. , Marcy, S. M. , Blum, M. , Jones, M. C. , Dagan, R. , Hansen, J. , Nalin, D. , & Rothstein, E. (2004). Fever after immunization: Current concepts and improved future scientific understanding. Clinical Infectious Diseases, 39(3), 389–394. 10.1086/422454 [DOI] [PubMed] [Google Scholar]
- Kumar, R. , Qureshi, H. , Deshpande, S. , & Bhattacharya, J. (2018). Broadly neutralizing antibodies in HIV‐1 treatment and prevention. Therapeutic Advances in Vaccines and Immunotherapy, 6(4), 61–68. 10.1177/2515135518800689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, D. T. S. , Samaranayake, L. P. , Leung, Y. Y. , & Neelakantan, P. (2020). Facial protection in the era of COVID‐19: A narrative review. Oral Diseases. 27(S3), 665–673. 10.1111/odi.13460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M. A. (2019). A comparison of plasmid DNA and mRNA as vaccine technologies. Vaccines, 7(2), 37. 10.3390/vaccines7020037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logunov, D. Y. , Dolzhikova, I. V. , Zubkova, O. V. , Tukhvatulin, A. I. , Shcheblyakov, D. V. , Dzharullaeva, A. S. , Grousova, D. M. , Erokhova, A. S. , Kovyrshina, A. V. , Botikov, A. G. , Izhaeva, F. M. , Popova, O. , Ozharovskaya, T. A. , Esmagambetov, I. B. , Favorskaya, I. A. , Zrelkin, D. I. , Voronina, D. V. , Shcherbinin, D. N. , Semikhin, A. S. , … Gintsburg, A. L. (2020). Safety and immunogenicity of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine in two formulations: Two open, non‐randomised phase 1/2 studies from Russia. The Lancet, 396(10255), 887–897. 10.1016/S0140-6736(20)31866-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrie, P. (2016). How disease affected the end of the bronze age. A history of disease in ancient times: More lethal than war, 61–101. 10.1007/978-3-319-28937-3_5 [DOI] [Google Scholar]
- Pardi, N. , Hogan, M. J. , Porter, F. W. , & Weissman, D. (2018). mRNA vaccines — a new era in vaccinology. Nature Reviews Drug Discovery, 17(4), 261–279. 10.1038/nrd.2017.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack, F. P. , Thomas, S. J. , Kitchin, N. , Absalon, J. , Gurtman, A. , Lockhart, S. , Perez, J. L. , Pérez Marc, G. , Moreira, E. D. , Zerbini, C. , Bailey, R. , Swanson, K. A. , Roychoudhury, S. , Koury, K. , Li, P. , Kalina, W. V. , Cooper, D. , Frenck, R. W. , Hammitt, L. L. , … Gruber, W. C. (2020). Safety and Efficacy of the BNT162b2 mRNA Covid‐19 Vaccine. New England Journal of Medicine, 383(27), 2603–2615. 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland, G. A. , Ovsyannikova, I. G. , Crooke, S. N. , & Kennedy, R. B. (2020). SARS‐CoV‐2 vaccine development: Current status. Mayo Clinic Proceedings, 95(10), 2172–2188. 10.1016/j.mayocp.2020.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy, M. N. , Minassian, A. M. , Ewer, K. J. , Flaxman, A. L. , Folegatti, P. M. , Owens, D. R. , Voysey, M. , Aley, P. K. , Angus, B. , Babbage, G. , Belij‐Rammerstorfer, S. , Berry, L. , Bibi, S. , Bittaye, M. , Cathie, K. , Chappell, H. , Charlton, S. , Cicconi, P. , Clutterbuck, E. A. , … Zizi, D. (2020). Safety and immunogenicity of ChAdOx1 nCoV‐19 vaccine administered in a prime‐boost regimen in young and old adults (COV002): A single‐blind, randomised, controlled, phase 2/3 trial. The Lancet, 396(10267), 1979–1993. 10.1016/S0140-6736(20)32466-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch, S. , Jasny, E. , Schmidt, K. E. , & Petsch, B. (2018). New vaccine technologies to combat outbreak situations. Frontiers in Immunology, 9, 1963. 10.3389/fimmu.2018.01963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero‐Maldonado, A. , Salazar‐González, J. A. , & Rosales‐Mendoza, S. . (2014). Plant‐based vaccines against Influenza. In Genetically engineered plants as a source of vaccines against wide spread diseases (pp. 129–139). 10.1007/978-1-4939-0850-9_7. [DOI] [Google Scholar]
- Sahin, U. , Muik, A. , Derhovanessian, E. , Vogler, I. , Kranz, L. M. , Vormehr, M. , Baum, A. , Pascal, K. , Quandt, J. , Maurus, D. , Brachtendorf, S. , Lörks, V. , Sikorski, J. , Hilker, R. , Becker, D. , Eller, A.‐K. , Grützner, J. , Boesler, C. , Rosenbaum, C. , … Türeci, Ö. (2020). COVID‐19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature, 586(7830), 594–599. 10.1038/s41586-020-2814-7 [DOI] [PubMed] [Google Scholar]
- Samaranayake, L. (2018). Essential microbiology for dentistry. 5th ed, Elsevier, 1–400. [Google Scholar]
- Samaranayake, L. (2020). COVID‐19 and dentistry: Perspectives of an unfolding pandemic. Dental Update, 47, 531–532. 10.12968/denu.2020.47.6.531 [DOI] [Google Scholar]
- Samaranayake, L. P. , & Anil, S. (2021). Understanding COVID‐19 vaccines, and Immunity. Dental Update, 48(2), 157–160. 10.12968/denu.2021.48.2.157 [DOI] [Google Scholar]
- Samaranayake, L. P. , & Fakhruddin, K. S. (2021). COVID‐19 Vaccines and Dentistry. Dental Update, 48, 76–81. [Google Scholar]
- Sanders, B. , Koldijk, M. , & Schuitemaker, H. (2014). Inactivated viral vaccines. Vaccine Analysis: Strategies, Principles, and Control, 45–80. 10.1007/978-3-662-45024-6_2 [DOI] [Google Scholar]
- Schlake, T. , Thess, A. , Fotin‐Mleczek, M. , & Kallen, K.‐J. (2012). Developing mRNA‐vaccine technologies. RNA Biology, 9(11), 1319–1330. 10.4161/rna.22269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn, A. L. (2013). Protective antiviral antibodies that lack neutralizing activity: Precedents and evolution of concepts. Current HIV Research, 11(5), 345–353. 10.2174/1570162x113116660057 [DOI] [PubMed] [Google Scholar]
- Speiser, D. E. , & Bachmann, M. F. (2020). COVID‐19: Mechanisms of vaccination and immunity. Vaccines (Basel), 8(3), 404. 10.3390/vaccines8030404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration (FDA) . (2020a). FDA briefing document for COVID‐19 vaccine EUA by Pfizer. 1‐53. [Google Scholar]
- U.S. Food and Drug Administration (FDA) . (2020b). Moderna COVID‐19 Vaccine EUA Letter of Authorization. 1‐9. [Google Scholar]
- U.S. Food and Drug Administration (FDA) . (2020c). Moderna COVID‐19 vaccine FDA briefing document. 1‐54. [Google Scholar]
- U.S. Food and Drug Administration (FDA) (2020d). Pfizer COVID‐19 EUA Letter of Authorization Reissued, 122320, 1–9. [Google Scholar]
- U.S. Food and Drug Administration (FDA) . (2021). Janssen Ad26.COV2.S Vaccine for the Prevention of COVID‐19. FDA VRBPAC‐02.26.21‐Meeting‐Briefing‐Document. 26 February 2021, 1‐62. [Google Scholar]
- Volgenant, C. M. C. , Persoon, I. F. , de Ruijter, R. A. G. , & de Soet, J. J. H. (2020). Infection control in dental health care during and after the SARS‐CoV‐2 outbreak. Oral Diseases. 23(S3), 674–683. 10.1111/odi.13408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Li, X. , Li, T. , Zhang, S. , Wang, L. , Wu, X. , & Liu, J. (2020). The genetic sequence, origin, and diagnosis of SARS‐CoV‐2. European Journal of Clinical Microbiology and Infectious Diseases, 39(9), 1629–1635. 10.1007/s10096-020-03899-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Peng, Y. , Xu, H. , Cui, Z. , & Williams, R. O. 3rd (2020). The COVID‐19 Vaccine Race: Challenges and Opportunities in Vaccine Formulation. American Association of Pharmaceutical Scientists, 21(6), 225. 10.1208/s12249-020-01744-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2015). Vaccine‐preventable diseases and vaccines. International Travel and Health ‐ CHAPTER, 6, 1–61. [Google Scholar]
- World Health Organization (WHO) (2017). Vaccines‐and‐trust. World Health Oraganization (WHO)/Europe, https://www.euro.who.int/__data/assets/pdf_file/0004/329647/Vaccines‐and‐trust.PDF, 1‐50 [Google Scholar]
- World Health Organization (WHO) . (2020a). SARS‐CoV‐2 Variants. Disease Outbreak News, World Health Organization. Updated 31st December 2020. https://www.who.int/csr/don/31‐december‐2020‐sars‐cov2‐variants/en/. 1‐2 [Google Scholar]
- World Health Organization (WHO) (2020b). Vaccine management and logistics support. World Health Organization (WHO) (p. 1‐15.). https://www.who.int/immunization/programmes_systems/supply_chain/resources/tools/en/ [Google Scholar]
- World Health Organization (WHO) . (2020c). World Health Organization ‐COVID‐19 candidate vaccine landscape. 1‐3. [Google Scholar]
- World Health Organization ‐report. (2021). Coronavirus disease 2019 (COVID‐19): situation report, January 2021. [Google Scholar]
- Zhang, C. , Maruggi, G. , Shan, H. , & Li, J. (2019). Advances in mRNA vaccines for infectious diseases. Frontiers in Immunology, 10, 594. 10.3389/fimmu.2019.00594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. , Zeng, H. , Gu, J. , Li, H. , Zheng, L. , & Zou, Q. (2020). Progress and prospects on vaccine development against SARS‐CoV‐2. Vaccines, 8(2), 153. 10.3390/vaccines8020153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Zeng, G. , Pan, H. , Li, C. , Hu, Y. , Chu, K. , Han, W. , Chen, Z. , Tang, R. , Yin, W. , Chen, X. , Hu, Y. , Liu, X. , Jiang, C. , Li, J. , Yang, M. , Song, Y. , Wang, X. , Gao, Q. , … Zhu, F. (2021). Safety, tolerability, and immunogenicity of an inactivated SARSCoV‐2 vaccine in healthy adults aged 18–59 years: a randomised, double‐blind, placebo‐controlled, phase 1/2 clinical trial. The Lancet Infectious diseases, 21, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, F.‐C. , Li, Y.‐H. , Guan, X.‐H. , Hou, L.‐H. , Wang, W.‐J. , Li, J.‐X. , Wu, S.‐P. , Wang, B.‐S. , Wang, Z. , Wang, L. , Jia, S.‐Y. , Jiang, H.‐D. , Wang, L. , Jiang, T. , Hu, Y. I. , Gou, J.‐B. , Xu, S.‐B. , Xu, J.‐J. , Wang, X.‐W. , … Chen, W. (2020). Safety, tolerability, and immunogenicity of a recombinant adenovirus type‐5 vectored COVID‐19 vaccine: A dose‐escalation, open‐label, non‐randomised, first‐in‐human trial. The Lancet, 395(10240), 1845–1854. 10.1016/S0140-6736(20)31208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer, C. , Corum, J. , & Wee, S. L. (2021). Coronavirus Vaccine Tracker. New York Times. Updated Feb. 1, 2021. https://www.nytimes.com/interactive/2020/science/coronavirus‐vaccine‐tracker.html. 1‐2. [Google Scholar]