Abstract
Background
The coronavirus disease 2019 (COVID‐19) pandemic has significantly impacted health care systems. However, to date, the trend of hospitalizations in the oncology patient population has not been studied, and the frequency of nosocomial spread to patients with cancer is not well understood. The objectives of this study were to evaluate the impact of COVID‐19 on inpatient oncology census and determine the nosocomial rate of COVID‐19 in patients with cancer admitted at a large academic center.
Materials and Methods
Medical records of patients with cancer diagnosed with COVID‐19 and admitted were reviewed to evaluate the temporal trends in inpatient oncology census during pre–COVID‐19 (January 2019 to February 2020), COVID‐19 (March to May 2020), and post–COVID‐19 surge (June to August 2020) in the region. In addition, nosocomial infection rates of SARS‐CoV‐2 were reviewed.
Results
Overall, the daily inpatient census was steady in 2019 (median, 103; range, 92–118) and until February 2020 (median, 112; range, 102–114). However, there was a major decline from March to May 2020 (median, 68; range, 57–104), with 45.4% lower admissions during April 2020. As the COVID‐19 surge eased, the daily inpatient census over time returned to the pre–COVID‐19 baseline (median, 103; range, 99–111). One patient (1/231, 0.004%) tested positive for SARS‐CoV‐2 13 days after hospitalization, and it is unclear if it was nosocomial or community spread.
Conclusion
In this study, inpatient oncology admissions decreased substantially during the COVID‐19 surge but over time returned to the pre–COVID‐19 baseline. With aggressive infection control measures, the rates of nosocomial transmission were exceedingly low and should provide reassurance to those seeking medical care, including inpatient admissions when medically necessary.
Implications for Practice
The COVID‐19 pandemic has had a major impact on the health care system, and cancer patients are a vulnerable population. This study observes a significant decline in the daily inpatient oncology census from March to May 2020 compared with the same time frame in the previous year and examines the potential reasons for this decline. In addition, nosocomial rates of COVID‐19 were investigated, and rates were found to be very low. These findings suggest that aggressive infection control measures can mitigate the nosocomial infection risk among cancer patients and the inpatient setting is a safe environment, providing reassurance.
Keywords: COVID‐19, Nosocomial rate, Cancer, Inpatient, Clinical outcomes
Short abstract
To understand the overall impact of COVID‐19 on health care delivery in the oncology setting, this study evaluated the inpatient oncology census, in comparison to historical data and infusion volume, at an institution with a high volume of COVID‐19 admissions.
Introduction
The emergence of coronavirus disease 2019 (COVID‐19) has dramatically altered the global landscape, including health care delivery. As a result of surge modeling and the need for urgent inpatient capacity, hospitals and physicians deferred nonurgent, non–COVID‐19–related visits and care. However, there is growing concern that patients who are seriously ill are also deferring care. A recent survey conducted by the American College of Emergency Physicians found that 80% of respondents were fearful of contracting COVID‐19 in the emergency room (ER), 29% delayed seeking medical care because of concerns of COVID‐19, and 73% worried about overstressing the health care system [1]. Data from Northern Italy indicate that hospitals have experienced a significant decrease in admissions related to acute coronary syndrome, while seeing an increase in mortality from causes not fully attributable to COVID‐19 [2].
Typically, transmission of SARS‐CoV‐2 occurs from symptomatic individuals with close contact through respiratory droplets or with contaminated surfaces. Time from exposure to symptom onset can be up to 14 days, and presymptomatic transmission has been documented [3]. These factors led many hospitals to adopt a no‐visitors policy in facilities to limit spread. However, a patient comes into contact with a number of health care workers during admission. Because subsets of patients (e.g., immunocompromised, diagnosed with hematological malignancies) have increased risk of nosocomial infection and greater susceptibility to serious illness because of SARS‐CoV‐2, the fear of contracting the disease may override their need to seek urgent care [4, 5, 6, 7]. However, to date, the trend of hospitalizations pre– and post–COVID‐19 surge in the oncology patient population has not been studied, and, outside of a single study from China (Wuhan) that found 28.6% (n = 8) of patients with cancer may have acquired COVID‐19 while hospitalized [8], the frequency of nosocomial spread to patients with cancer is not well described.
As it is imperative to understand the overall health impacts of COVID‐19 and the risks of infection during hospitalization, the primary objectives of this study were to (a) evaluate the inpatient oncology census in comparison with historical data and infusion volume at an institution with a high volume of COVID‐19 admissions and (b) determine the nosocomial rate of COVID‐19 in patients with cancer admitted at a large academic center.
Materials and Methods
Patients with a known diagnosis of cancer who were hospitalized at Massachusetts General Hospital (MGH) in Boston, MA, with a SARS‐CoV‐2–positive test result between March 1, 2020, and September 1, 2020, were retrospectively identified. A comprehensive COVID Cancer Research (CCR) patient registry was created using the database software REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN), a management platform used worldwide for information storage on research studies. The study was approved by the Partners Human Research Committee, the institutional review board of Partners HealthCare, before any research was conducted.
Data Collection and Analysis
Medical records of patients with cancer who were diagnosed with COVID‐19 and admitted to MGH were reviewed, and data were entered into the CCR registry. Details regarding the COVID‐19 test (SARS‐CoV‐2 polymerase chain reaction) for every patient were also collected to determine timing of infection in relation to inpatient admission and to assess nosocomial rate. Furthermore, we chart reviewed all MGH Cancer Center (MGHCC) patients with confirmed COVID‐19 (between March 1, 2020, and September 1, 2020) to determine if they had been recently hospitalized in the 14 days prior to the diagnosis. In addition, we collected operational data on the volume of new patients, volume of inpatient and outpatient infusions, number of patients with an emergency department visit at MGH, and number of individuals transferred from outside hospital to our institution from August 14, 2019, to August 14, 2020. Statistical analyses were performed using Stata, version 15.0 (StataCorp, College Station, TX), and the corresponding results were graphed using Microsoft Excel.
Results
Inpatient Oncology Census
We first evaluated the temporal trends in inpatient oncology census during pre–COVID‐19 (January 2019 to February 2020), COVID‐19 surge (March to May 2020), and post–COVID‐19 surge (June to August 2020) at MGH. Overall, the daily inpatient census was steady in 2019 with a median of 103 patients (range, 92–118), as well as in January–February 2020 with a median of 112 patients (range, 102–114). However, there was a major decline in daily inpatient census from March to May 2020, with a median of 68 patients (range, 57–104), coinciding with the peak COVID‐19 surge, as depicted in Figure 1.
Figure 1.
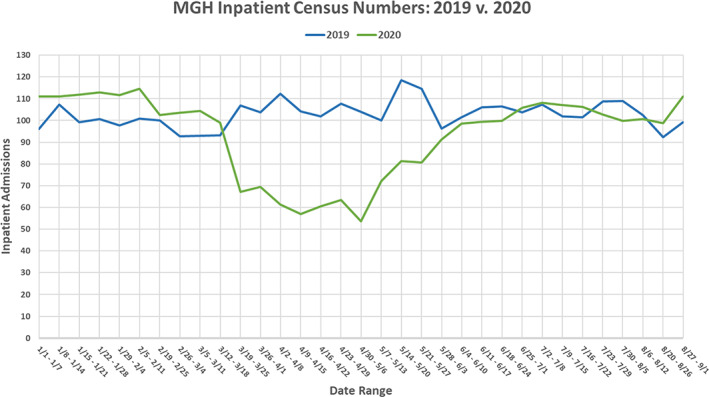
Comparing the weekly inpatient census numbers of patients with cancer at Massachusetts General Hospital between 2019 and 2020. Data exhibit a steep drop in the 2020 inpatient numbers following the beginning of the COVID‐19 quarantine. Abbreviations: MGH, Massachusetts General Hospital; v, versus.
April 2020 witnessed the peak of the COVID‐19 surge at our hospital, and the number of oncology patients with COVID‐19 was also high during this month (Fig. 2). In comparison, the general inpatient oncology census was 45.4% lower during the first week of April 2020 (April 2–April 8), as compared with a similar time frame in 2019. Likewise, the inpatient census was 41.1% lower during the last week of April 2020 (April 23–April 29), as compared with a similar time frame in 2019.
Figure 2.
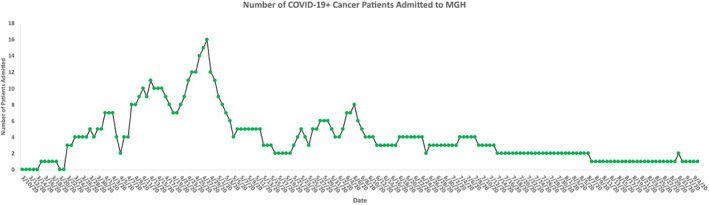
Displaying the number of admitted COVID‐19 positive patients with cancer at Massachusetts General Hospital. Data show a steady decline since the observed peak on April 28th, 2020. Abbreviation: MGH, Massachusetts General Hospital.
As the COVID‐19 surge eased in subsequent months in the Boston region, the daily inpatient census increased and returned to the pre–COVID‐19 baseline. The median inpatient census was 101 from June to August 2020, which was similar to the census from June to August 2019 (median, 103; range, 99–111).
Operational Data
Since inpatient admissions could be impacted by number of patients receiving inpatient and outpatient chemotherapy (Fig. 3; supplemental online Fig. 1A), number of new patients diagnosed with cancer (supplemental online Fig. 1D), established‐patient follow‐up or emergency department volume (supplemental online Fig. 1B), and number of outside hospital transfers (supplemental online Fig. 1C), we then evaluated the trends in these areas to assess whether any change could account for the major decline in inpatient census from March to June 2020. Although there was a decline in outpatient infusion volume during the months of March to June 2020, there is no clear trend between outpatient infusion volume decrease and inpatient admissions during the same time frame (Fig. 3). During this same time frame, however, there was a decrease in inpatient infusions (supplemental Fig. 1A), emergency department volume, and outside hospital transfers. Overall, established‐patient volume year to date from January 1 through August 31 was down 1% in 2020 (146,899 visits in 2019, 145,738 visits in 2020) and new‐patient volume year to date from January 1 through August 31 was down 21% (22,706 new patients in 2019, 17,915 patients in 2020).
Figure 3.
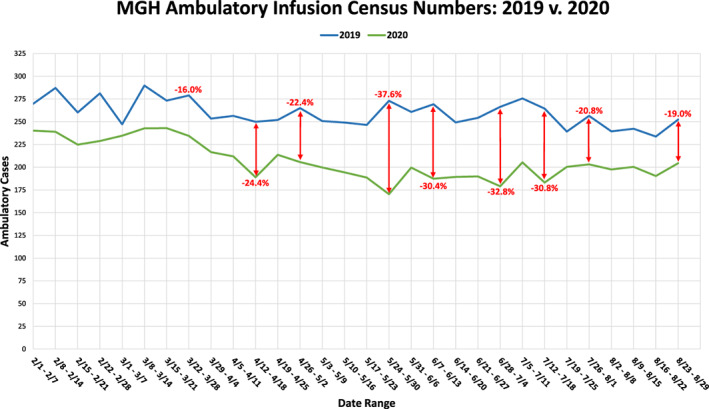
Comparing the weekly ambulatory infusion census numbers of patients with cancer at Massachusetts General Hospital between 2019 and 2020. Data show an overall decrease in census numbers in 2020 but also present a drop in ambulatory infusion numbers shortly following the beginning of the COVID‐19 pandemic quarantine. Abbreviations: MGH, Massachusetts General Hospital; v, versus.
Nosocomial Infection
Given the strong relationship between COVID‐19 surge and decline in inpatient oncology census, we next evaluated the rate of nosocomial infections to investigate whether there was an objective risk of COVID‐19 exposure and infection while hospitalized that could have prompted patients (and providers) to avoid inpatient admissions.
We evaluated the temporal relationship between COVID‐19 positivity and inpatient admission among patients with cancer at MGHCC (n = 231). Of note, 57 individuals were hospitalized within 14 days of positive SARS‐CoV‐2 test results (Fig. 4). Fifty‐six individuals were admitted within 1 week after a positive test result or were tested within 24 hours of admission. Three of these patients tested postive 24 hours after admission, which argues against nosocomial infection since the incubation period of COVID‐19 is 5–7 days [9]. One individual (1/231, 0.004%) tested positive for SARS‐CoV‐2 13 days after a recent hospitalization for a non–COVID‐19–related illness. It is unclear if this was a case of nosocomial or community spread. These data indicate that there was an exceedingly low nosocomial infection rate.
Figure 4.
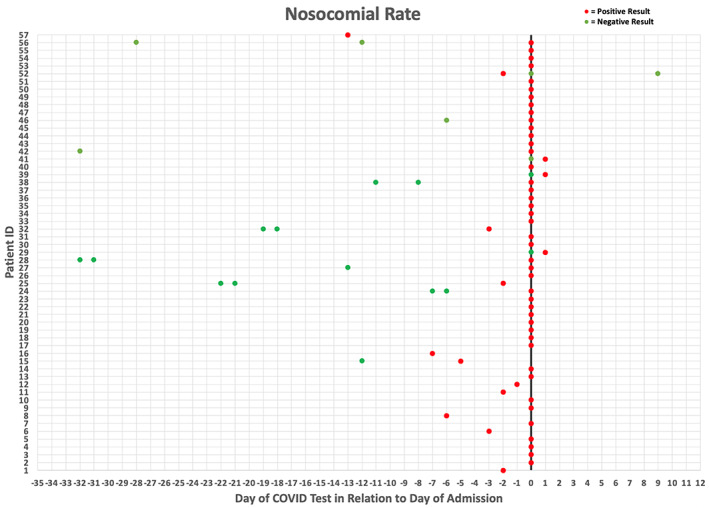
Displaying COVID‐19 test results in relation to the patient's day of admission (x = 0) to Massachusetts General Hospital. Red dots designate a positive test result, and green dots designate a negative test result. Data indicate that admitted patients were testing positive for COVID‐19 prior to admission or on the day of admission, which implies that patients were not acquiring nosocomial COVID‐19 infections. Abbreviation: ID, identification.
Discussion
In this study, we found that the number of patients with cancer who were admitted to the hospital decreased substantially during the COVID‐19 surge, but as the surge eased in subsequent months, the daily inpatient census started to increase and returned close to the pre–COVID‐19 baseline. Our data suggest that the decrease in inpatient admissions was not due to a decrease in outpatient infusion volume alone or one clear driving factor. We observed a general decline in the number of new cancer diagnoses, the volume of patients with cancer being admitted for inpatient treatment and from the emergency department, and a decrease in the volume of patients transferred from outside hospitals, suggesting that there are multiple factors that played a role in the decline in inpatient census. Importantly, and as evidenced by the decreased new‐patient volume (supplemental online Fig. 1), fear and perception among patients (and providers) to avoid inpatient admissions in a hospital with a high volume of patients with COVID‐19 could have also played a role. However, the exceedingly low rate of nosocomial infections in our cohort does not support this latter concern.
A decline in patients seeking out or presenting for care has also been reported in care settings other than cancer [2, 10]. A recent ER study from five health care systems across five states found that the number of ER visits decreased by 60% in areas where COVID‐19 was most severe [11]. A retrospective survey of 847 patients from 13 gastroenterology centers in Northern Italy found that 29.4% of patients did not to show up for their endoscopy on their scheduled date. During the 3‐week study, the percentage of missed appointments progressively increased from 15.1% at the beginning to 48.2% at the end [12]. A Turkish study found a significant drop in the number of new cancer diagnosis, use of interventional procedures, and receipt of palliative care services [13]. Several other studies have found a precipitous decline in screening for breast, prostate, and colorectal cancer during the peak of COVID‐19 [14, 15].
Currently, there is a dearth of high‐quality data exploring the real or long‐term effect on outcomes in patients who have a delay in cancer diagnosis or treatment, with most of these studies being case series and observational with limited follow‐up. Additionally, few studies have quantified inpatient oncology admissions and potential avoidance of inpatient hospitalization during the COVID‐19 pandemic. Furthermore, many of the findings seem to vary depending on the type of cancer and setting of the reporting. However, there is great concern that these delays could have significant consequences. In a recent national, population‐based modeling study in the U.K., researchers estimated that across four major tumor types (breast, colorectal, lung, and esophagus), 3,291–3,621 avoidable deaths and 59,204–63,299 years of life lost will be attributable to delays in cancer diagnosis alone as a result of the COVID‐19 lockdown [16]. The impact of delayed inpatient admissions is not known to the best of our knowledge, and this is an area that warrants further research.
Various COVID‐19 registries have evaluated why patients are delaying their medical care during the pandemic. In addition to the public being encouraged to stay at home and some governments instituting nationwide lockdowns, another possible explanation is fear. Patients are concerned about coming into contact with those infected with SARS‐CoV‐2 if they have to be admitted to the hospital or come to the clinic for treatment. A study investigating how elevated anxiety and fear during the pandemic affected chemotherapy adherence in patients with cancer found that the computerized tomography (CT) scan postponement rates before and after COVID‐19 were 11.6% and 14.2%, respectively (p = .017). COVID‐19 fear and anxiety (COV‐FA) was identified as the third most frequent reason for CT scan postponement (after neutropenia and thrombocytopenia). After telemedicine was implemented and included information about rates of COVID‐19 in the health care center, the rate of COV‐FA–related CT scan postponement decreased significantly (4.6% after telemedicine vs. 17.4% before telemedicine, p = .012), indicating that it is important to share information with the public to alleviate concerns [17]. In a survey study based on WhatsApp messages from Italian oncology patients, older patients (≥75 years) and those with poor performance status were most worried about COVID‐19, which demonstrated that more fragile patients and their families were the most concerned [18]. Fear was the dominant emotion identified. A small percentage of patients asked for withdrawal from treatment, indicating a greater fear of acquiring COVID‐19 than of their cancer progressing. Media coverage of sporadic outbreaks further compounded the fear and anxiety.
Although fear of acquiring COVID‐19 in the hospital is understandable, with adequate preventive measures the actual rate of nosocomial infection appears to be low. In our study, none of the patients with cancer admitted to the hospital with COVID‐19 acquired the infection while hospitalized. There was one individual who was discharged 13 days prior to his COVID‐19–positive test result, and the method of spread was unclear. This low risk of viral transmission has also been observed in several other studies involving health care workers (HCWs). In a study of 60 HCWs exposed ≤2 meters for ≥15 minutes, or during aerosol‐generating procedures (AGPs), following ≥106 unique close contact exposures including 12 contacts during AGPs with a nonisolated patient with COVID‐19, none of the HCWs tested positive for SARS‐CoV‐2 RNA or developed antibodies [19]. Similarly, in a study of 545 asymptomatic HCWs who volunteered to be tested for SARS‐CoV‐2 to determine the rates of asymptomatic viral carriage and seroprevalence of SARS‐CoV‐2 antibodies, the rates were found to be 2.4% (13/545) and 24.4% (126/516), respectively [20]. To characterize SARS‐CoV‐2 transmission to health care providers (HCPs) within and outside the medical workplace, an international case‐control study of 1,130 HCPs (244 cases with laboratory‐confirmed SARS‐CoV‐2, 886 controls healthy throughout the pandemic) reported through an online survey that transmission was associated with non–aerosol‐generating contact and multiple extraoccupational exposures, whereas exposures associated with proper use of appropriate personal protective equipment (PPE) were protective [21]. With the implementation of active and enhanced surveillance with progressively wider screening criteria during the evolution of the COVID‐19 pandemic, hospitals in Hong Kong achieved zero nosocomial transmission in HCWs and patients within the first 6 weeks of investigation [22].
Our low rate of nosocomial infection was similar to what was recently reported from one of our partner hospitals [23]. Over the first 12 weeks of the pandemic, Brigham and Women's Hospital cared for more than 9,000 patients, including approximately 700 with COVID‐19, and identified only two patients who likely acquired the infection in the hospital, including one who was most likely infected by a spouse before visitor restrictions and universal masking [23]. However, our study included only patients with cancer, who are a more vulnerable cohort. With rigorous infection control measures, which included implementation of universal face mask policies for all patients and staff, restrictions on visitors to the hospital, and transition of all nonurgent oncology visits to virtual care, our cohort study showed that nosocomial COVID‐19 was rare even among an at‐risk population during the height of the pandemic in the region. However, this is with adequate PPE and precaution, and there is concern that outcomes might not be the same with a future surge, particularly in the setting of an increasingly tired and distressed workforce [24].
Our study has several limitations. This was a single‐center study involving a large academic institution with significant resources. Our hospital implemented aggressive infection control measures early on during the pandemic and updated and enforced them rigorously. Thus, our findings may not be generalizable to smaller, under‐resourced institutions. Furthermore, we only studied patients with cancer who were admitted to Massachusetts General Hospital with COVID‐19, and we were unable to track our patients who may have been admitted to other hospitals around the state or country.
Conclusion
Patients with cancer are considered to be a high‐risk population for SARS‐CoV‐2 infection because of their immunocompromised state and multiple comorbidities; they also tend to be older. Thus, it is not surprising that our rates of cancer hospital admission and infusion visits declined dramatically after the appearance of COVID‐19. However, delays in the diagnosis of or treatment for cancer or treatment complications can have detrimental effects on outcomes. In our hospital, where aggressive infection control measures were employed, the low rate of nosocomial transmission should provide reassurance to patients with cancer who are concerned about contracting COVID‐19 if they come to the hospital or clinic for cancer care. It is clear that the lowest rates of transmission occur in hospitals with the most effective infection control measures, and this provides an important public health message for the future. The low rate of viral transmission should also help providers encourage their patients with cancer to seek medical care, including inpatient admissions when medically necessary.
Author Contributions
Conception/design: Leyre Zubiri, Rachel P. Rosovsky, Meghan J. Mooradian, A.J. Piper‐Vallillo, Justin F. Gainor, Ryan J. Sullivan, Daniel Marte, Genevieve M. Boland, Xin Gao, Ephraim P. Hochberg, David P. Ryan, Corey McEwen, Minh Mai, Tanya Sharova, Tara E Soumerai, Aditya Bardia, Kerry L. Reynolds
Provision of study material or patients: Leyre Zubiri, Rachel P. Rosovsky, Meghan J. Mooradian, A.J. Piper‐Vallillo, Justin F. Gainor, Ryan J. Sullivan, Daniel Marte, Genevieve M. Boland, Xin Gao, Ephraim P. Hochberg, David P. Ryan, Corey McEwen, Minh Mai, Tanya Sharova, Tara E Soumerai, Aditya Bardia, Kerry L. Reynolds.
Collection and/or assembly of data: Leyre Zubiri, Rachel P. Rosovsky, Meghan J. Mooradian, A.J. Piper‐Vallillo, Justin F. Gainor, Ryan J. Sullivan, Daniel Marte, Genevieve M. Boland, Xin Gao, Ephraim P. Hochberg, David P. Ryan, Corey McEwen, Minh Mai, Tanya Sharova, Tara E Soumerai, Aditya Bardia, Kerry L. Reynolds
Data analysis and interpretation: Leyre Zubiri, Rachel P. Rosovsky, Meghan J. Mooradian, A.J. Piper‐Vallillo, Justin F. Gainor, Ryan J. Sullivan, Daniel Marte, Genevieve M. Boland, Xin Gao, Ephraim P. Hochberg, David P. Ryan, Corey McEwen, Minh Mai, Tanya Sharova, Tara E Soumerai, Aditya Bardia, Kerry L. Reynolds
Manuscript writing: Leyre Zubiri, Rachel P. Rosovsky, Meghan J. Mooradian, A.J. Piper‐Vallillo, Justin F. Gainor, Ryan J. Sullivan, Daniel Marte, Genevieve M. Boland, Xin Gao, Ephraim P. Hochberg, David P. Ryan, Corey McEwen, Minh Mai, Tanya Sharova, Tara E Soumerai, Aditya Bardia, Kerry L. Reynolds
Final approval of manuscript: Leyre Zubiri, Rachel P. Rosovsky, Meghan J. Mooradian, A.J. Piper‐Vallillo, Justin F. Gainor, Ryan J. Sullivan, Daniel Marte, Genevieve M. Boland, Xin Gao, Ephraim P. Hochberg, David P. Ryan, Corey McEwen, Minh Mai, Tanya Sharova, Tara E Soumerai, Aditya Bardia, Kerry L. Reynolds
Disclosures
Leyre Zubiri: Merck (C/A); Rachel P. Rosovsky: Bristol‐Myers Squibb, Janssen (RF—institutional), Bristol‐Myers Squibb, Janssen (C/A); Meghan J. Mooradian: AstraZeneca, Nektar Therapeutics, Immunai, Catalyst Pharmaceuticals (C/A, H); Ryan J. Sullivan: Asana Biosciences, AstraZeneca, Bristol‐Myers Squibb, Eisai, Merck, Novartis, OncoSec, Pfizer, Replimune (C/A), Merck (RF); Genevieve M. Boland: Palleon Pharmaceuticals, Olink Proteomics, Takeda Oncology (RF), Novartis (H), Novartis, Nectar Therapeutics (SAB); Aditya Bardia: bioTheranostics; Genentech (C/A), Genentech/Roche, Immunomedics, Innocrin Pharma, Merck, Novartis, Pfizer, Radius Health, Radius Pharma, Sanofi, Spectrum Pharmaceuticals, bioTheranostics (RF); Ephraim P. Hochberg: Leuko (OI), TRAPelo (C/A); David P. Ryan: Acworth Pharmaceuticals, Thrive Earlier Detection, MPM Capital (OI), MPM Capital, Gritstone Oncology, Maverick Therapeutics, Johns Hopkins University Press, Uptodate, McGraw Hill (H), SU2C (RF), Boerginer Ingelheim, Iteos (Other—legal fees). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Figure S1 Supplementary Figure
Acknowledgments
Dr. Leyre Zubiri would like to acknowledge Spanish Society of Medical Oncology Sociedad Española de Oncología Médica for her grant for a 2‐year translational project at the Massachusetts General Hospital (MGH) Cancer Center. Daniel Marte would like to acknowledge the COVID Corps Biomedical Research Internship Program for his stipend for research at MGH.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. American College of Emergency Physicians . Public Poll: Emergency Care Concerns Amidst COVID‐19. American College of Emergency Physicians Web site. Available at https://www.emergencyphysicians.org/article/covid19/public-poll-emergency-care-concerns-amidst-covid-19. Accessed June 19, 2020.
- 2. De Filippo O, D'Ascenzo F, Angelini F et al. Reduced rate of hospital admissions for ACS during Covid‐19 outbreak in Northern Italy. N Engl J Med 2020;383:88–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kronbichler A, Kresse D, Yoon S et al. Asymptomatic patients as a source of COVID‐19 infections: A systematic review and meta‐analysis. Int J Infect Dis 2020;98:180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. He W, Chen L, Yuan G et al. COVID‐19 in persons with haematological cancers. Leukemia 2020;34:1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cook G, John Ashcroft A, Pratt G et al. Real‐world assessment of the clinical impact of symptomatic infection with severe acute respiratory syndrome coronavirus (COVID‐19 disease) in patients with multiple myeloma receiving systemic anti‐cancer therapy. Br J Haematol 2020;190:e83–e86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. El‐Sharif A, Elkhatib WF, Ashour HM. Nosocomial infections in leukemic and solid‐tumor cancer patients: Distribution, outcome and microbial spectrum of anaerobes. Future Microbiol 2012;7:1423–1429. [DOI] [PubMed] [Google Scholar]
- 7. Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol 2009;10:589–597. [DOI] [PubMed] [Google Scholar]
- 8. Zhang L, Zhu F, Xie L et al. Clinical characteristics of COVID‐19‐infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann Oncol 2020;31:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qin J, You C, Lin Q et al. Estimation of incubation period distribution of COVID‐19 using disease onset forward time: A novel cross‐sectional and forward follow‐up study. Sci Adv 2020;6:eabc1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baldi E, Sechi GM, Mare C et al. Out‐of‐hospital cardiac arrest during the COVID‐19 outbreak in Italy. N Engl J Med 2020;383:496–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeffery MM, D'Onofrio G, Paek H et al. Trends in emergency department visits and hospital admissions in health care systems in 5 states in the first months of the COVID‐19 pandemic in the US. JAMA Intern Med 2020;180:1328–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Armellini E, Repici A, Alvisi C et al. Analysis of patients attitude to undergo urgent endoscopic procedures during COVID‐19 outbreak in Italy. Dig Liver Dis 2020;52:695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guven DC, Aktas BY, Aksun MS et al. COVID‐19 pandemic: Changes in cancer admissions. BMJ Support Palliat Care 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14. D'Ovidio V, Lucidi C, Bruno G et al. Impact of COVID‐19 pandemic on colorectal cancer screening program. Clin Colorectal Cancer 2021;20:e5–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Warner ET, Restrepo E, Benjamin C et al. Patient‐reported impact of the COVID‐19 pandemic on breast cancer screening, diagnosis, and treatment: A national survey. Clin Cancer Res 2020;26(suppl 18):S11‐02a. [Google Scholar]
- 16. Maringe C, Spicer J, Morris M et al. The impact of the COVID‐19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population‐based, modelling study. Lancet Oncol 2020;21:1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karacin C, Bilgetekin I, Basal FB et al. How does COVID‐19 fear and anxiety affect chemotherapy adherence in patients with cancer. Future Oncol 2020;16:2283–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gebbia V, Piazza D, Valerio MR et al. Patients with cancer and COVID‐19: A WhatsApp messenger‐based survey of patients’ queries, needs, fears, and actions taken. JCO Glob Oncol 2020;6:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Basso T, Nordbo SA, Sundqvist E et al. Transmission of infection from non‐isolated patients with COVID‐19 to health care workers. J Hosp Infect 2020;106:639–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shields A, Faustini SE, Perez‐Toledo M et al. SARS‐CoV‐2 seroprevalence and asymptomatic viral carriage in healthcare workers: A cross‐sectional study. Thorax 2020;75:1089–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lentz RJ, Colt H, Chen H et al. Assessing COVID‐19 transmission to healthcare personnel: The global ACT‐HCP case‐control study. Infect Control Hosp Epidemiol 2020;42:381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng VCC, Wong SC, Chen JHK et al. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID‐19) due to SARS‐CoV‐2 in Hong Kong. Infect Control Hosp Epidemiol 2020;41:493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rhee C, Baker M, Vaidya V et al. Incidence of nosocomial COVID‐19 in patients hospitalized at a large US academic medical center. JAMA Netw Open 2020;3:e2020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cipolotti L, Chan E, Murphy P et al. Factors contributing to the distress, concerns, and needs of UK Neuroscience health care workers during the COVID‐19 pandemic. Psychol Psychother 2021;94(suppl 2):536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Figure S1 Supplementary Figure


