Abstract
Pontine warning syndrome (PWS) is a condition characterized by crescendo transient ischemic attacks due to pontine ischemia. The reported case described a 72-year-old woman who presented repetitive sudden episodes of double vision, impaired balance, slurred speech and right-sided weakness. Neurological deficits lasted a few minutes-hours and disappeared during the first seven days after onset. On the 1st day, MRI revealed acute left paramedian pontine infarction with focal swelling. Supra-aortic vessel imagining revealed bilateral internal carotid stenosis of 50%; hypoplasia of the left vertebral artery. On the 7th day, MRI showed a tissue swelling reduction, and from that day, she had no symptoms. These clinical and radiological features were suggestive of PWS. Our patient presented a particular vascular pattern that could favour symptoms fluctuation. We performed a close MRI follow up and it allowed us to observe a clinical stabilization in association with edema reduction.
Keywords: Pontine warning syndrome, Acute pontine ischemia, Vertebral artery hypoplasia, Carotid stenosis, MRI follow up
Highlights
-
•
Pontine warning syndrome (PWS) is due to paramedian pontine infarction.
-
•
Supra-aortic vessels hypoplasia and stenosis could have a determinant role in PWS.
-
•
A close MRI follow up could be helpful to predict clinical stability in PWS.
Pontine warning syndrome, acute pontine ischemia, vertebral artery hypoplasia, carotid stenosis, MRI follow up
1. Introduction
Pontine warning syndrome (PWS) is a rare condition, characterized by crescendo transient ischemic attacks, presenting with recurrent stereotyped episodes of motor weakness, sensory dysfunction, dysarthria, or ophthalmoplegia due to pontine ischemia [1]. The first case was described by Farrar et al. in 1993 [2], the terms was coined by Saposnik et al in 2008 [3], and it represents a diagnostic and therapeutic challenge. The hypothesized mechanism is a penetrating basilar artery branch occlusion at the origin. We reported the case of a patient admitted to our department focusing on the importance of the vascular pattern and the radiological follow up.
2. Case report
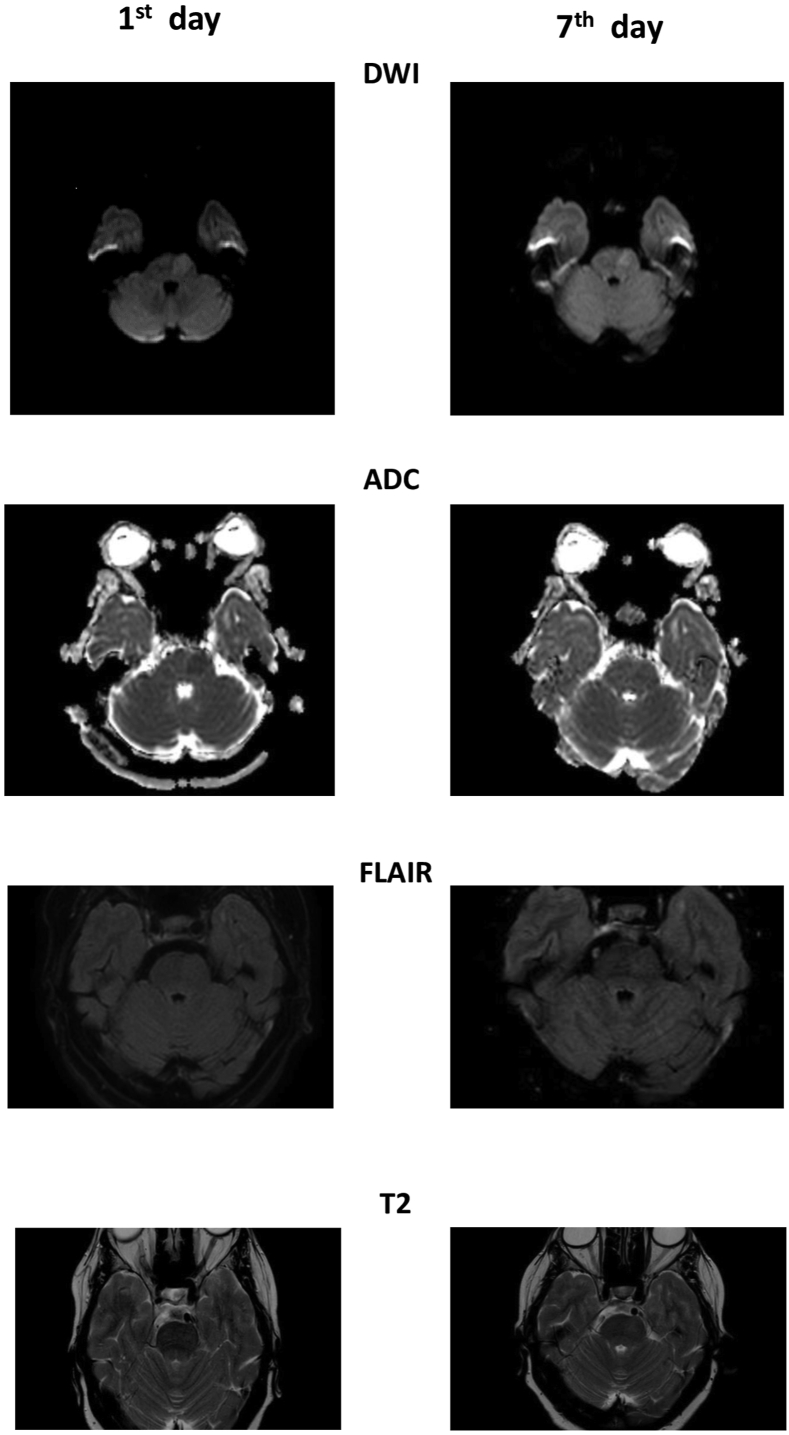
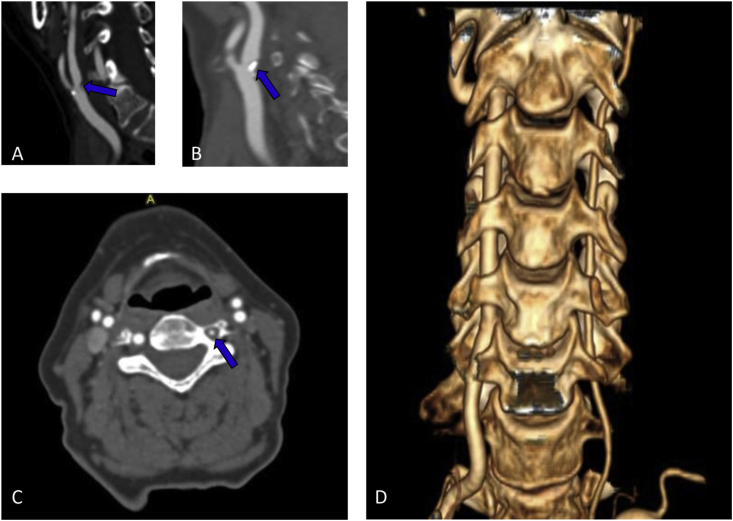
A 72-year-old woman experienced a sudden episode of double vision, impaired balance, slurred speech and right-sided weakness. Her medical history included hypertension and dyslipidemia. The patient did not take any drugs. In the past, she had first taken simvastatin and then ezetimibe, both ones were suspended due to myalgia. The patient had also stopped zofenopril and then amblodipine because of hypotensive episodes. Neurological deficits lasted a few minutes to reappear and resolve again during ambulance transportation. At admission, neurological examination was negative, and the woman presented a blood pressure (BP) of 175/115 mmHg, a sinus rhythm at 85 beats for a minute, and brain computed tomography scan (CT) did not show any acute ischemic or hemorrhagic lesion. After 2 hours, she developed right deviation of head and eyes, diplopia, mild right-sided hemiparesis, and dysarthria, National Institutes of Health Stroke Scale (NIHSS) 7. She underwent a brain Magnetic Resonance Imaging Scan (MRI) which revealed acute left paramedian pontine infarction (PPI) (Figure 1) with focal brain swelling, Classification of Cerebral oedema (COED) 1. The acute lesion was hyperintense in Diffusion-Weighted Imaging (DWI), slightly hyperintense in T2 and Fluid Attenuated Inversion Recovery (FLAIR), hypointense in Apparent Diffusion Coefficient (ADC). The intravenous thrombolysis was not performed because she was out of the therapeutic window. Her symptoms disappeared within an hour, and during the following days, she presented fluctuations varying from normal neurological examination to a clinical picture characterized by diplopia, dysarthria, and moderate right-sided hemiparesis. During hospitalization, she presented a high value of BP, and we noted rapid BP drops were associated with neurological worsening. An electroencephalogram did not show epileptic activity during an attack. The patient underwent an electrocardiogram, Holter electrocardiogram, transthoracic echocardiogram, and transcranial doppler with bubble study which excluded a cardioembolic etiology. Supra-aortic vessels were studied by color-doppler-ultrasound, angio-MRI, and angio-CT which revealed bilateral atherosclerotic plaques at the carotid bifurcations that continued in internal carotid arteries (ICA), determining stenosis of 50%; bilateral ICA tortuosity; hypoplasia of left vertebral artery (Figure 2). On the 7th day of hospitalization, a second MRI showed a tissue swelling reduction (Figure 1), and from that day, she had no symptoms fluctuation. On the 12th day, she was discharged at home with a normal neurological examination. The prescribed therapy was valsartan 20 mg, clopidogrel 75 mg and atorvastatin 20 mg (dose used for precedent myalgia on ezetimibe and simvastatin). At 1, 3, and 6 months follow-up visit neurological examination was negative, and she did not present episodes of focal neurological deficit. Written informed consent was obtained from the patient for the publication of this case report and any accompanying investigation results and imaging.
Figure 1.
Pontine ischemic lesion on Magnetic Resonance Imaging. DWI: Diffusion Weighted Imaging; FLAIR: Fluid Attenuation Inversion Recovery.
Figure 2.
Supra-aortic vascular pattern on Computed Tomography Angiography (CTA). (A) Right internal carotid artery stenosis; (B) Left internal carotid artery stenosis; (C) Left vertebral artery hypoplasia; (D) Vertebral arteries 3d reconstruction.
3. Discussion
These clinical and radiological features were suggestive of PWS. It is characterized by recurrent stereotyped episodes of motor weakness, sensory dysfunction, dysarthria, or ophthalmoplegia due to acute PPI.
The main risk factors are arterial hypertension, dyslipidemia, and diabetes mellitus [1, 4]. In Muengtaweepongsa et al. case series, the largest to date, every patient had hypertension [1]. Our patient suffered from both arterial hypertension and dyslipidemia. These elements play an important role in small vessel disease that should be at the base of PWS [1, 4]. The pathophysiological mechanism underlying PWS indeed is not very clear, the most accredited mechanism is a penetrating basilar artery branch occlusion at the origin, but no case in the literature shows a similar occlusion on radiological exams. The antero-medial terminal branches of the vertebral and basilar artery nourish corticospinal tract fibres, bilateral medial longitudinal fasciculus, medial lemniscus, and abducens nucleus in the pons [5]. A blood flow alteration could determine an PPI and the relative neurological manifestations. The intermittent clinical manifestations could be related to a local hemodynamic derangement [4, 6]. BP has a determinant function: these patients generally present elevated value of BP, and a rapid lowering is often associated with a neurological worsening [1], as our report. The single attack generally has a short duration, the paroxysmal character could suggest an epileptic seizure but electroencephalogram was normal in our patient and in other reported cases [7, 8]. The fluctuation of neurological deficits lasts from 1 to 7 days in the most cases [1]. CT fails to detect a pontine ischemic lesion, especially in acute setting [1]. MRI on the admission shows a PPI [3, 6, 7, 9, 10, 11] that could also disappear in early follow up, on the 1st-3rd day [9, 10], but a permanent ischemic lesion is generally visible after the 3rd-4th day [2, 4, 7, 8, 9, 11, 12, 13, 14, 15]. A disease similar to PWS was capsular warning syndrome, neuroimaging could show an internal capsule ischemia in this condition. Symptoms as ophtalmoplegia, diplopia, ataxia, and dizziness, if any, could also help to differentiate PWS from a capsular warning syndrome. Regarding therapy in PWS, intravenous thrombolysis was not administered in many cases because of diagnosis delay. The few undergone this treatment showed heterogeneous results but there were no hemorrhagic complications [4]. Single or dual antiplatelet therapy, heparin and statin were differently used but no conclusive data were obtained [6]. The prognosis indeed remains extremely variable from a fixed impairment to complete recovery without further ischemic attacks.
Our patient presented a particular vascular pattern with both stenosis and tortuosity of ICA and hypoplasia of a vertebral artery (HVA), it could favour symptoms fluctuation. In particular, Delcker et al. showed ICA stenosis favour vertebro-basilar TIA [16], and different articles associated HVA with posterior stroke, under certain diameter of the VA the blood flow is reduced leading to an unbalanced hemodynamic and an inadequate blood supply to the brain [17, 18, 19]. In literature other vascular patterns were also associated with PWS but they interested only the posterior circle: Algahtani et al. described a patient with an irregularity involving the left vertebral artery and basilar artery with mild luminal stenosis [8]; Muengtaweepongsa et al. found a basilar artery stenosis in two patients and a basilar artery dolichoectasia in three [1]; Chan and Silver's case had a severe mid-basilar stenosis associated with absence of posterior communicating arteries [12], Farrar et al. and Nadarajan et al. patients had a left HVA [2, 15]. In the light of the above, a transcranial doppler monitoring could be useful to observe a reduced blood flow in the cerebral arteries and/or a possible change during hospitalization. It could explain neurological fluctuations but is a speculative point of view. Furthermore, the acute setting and the frequent fast resolution of the symptoms represent an important limitation.
Another element which we would highlight is the importance of a close radiological follow up. Our patient presented fluctuations until the 7th day of hospitalization according to Muengtaweepongsa S et al. study [1] and we performed the first MRI on admission, the second one on the 7th day. It allowed us to observe a stabilization of clinical symptoms in association with tissue swelling reduction. We could argue that a reduction in edema and local inflammation could improve local perfusion.
4. Conclusion
The presented case permitted to focus same features that could have a determinant role in PWS. Our patients had hypoplasia of the left vertebral artery, which corresponded with the infarct side, she presented also an ICA stenosis of 50% and bilateral ICA tortuosity that could favour symptom fluctuations. A close MRI follow up allowed us to observe a stabilization of clinical symptoms in association with pontine edema reduction. These elements should be considered and evaluated in PWS cases.
Declarations
Author contribution statement
All authors listed have significantly contributed to the investigation, development and writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Muengtaweepongsa S., Singh N.N., Cruz-Flores S. Pontine warning syndrome: case series and review of literature. J. Stroke Cerebrovasc. Dis. 2010;19:353–356. doi: 10.1016/j.jstrokecerebrovasdis.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Farrar J., Donnan G.A. Capsular warning syndrome preceding pontine infarction. Stroke. 1993;24:762. doi: 10.1161/01.str.24.5.762. [DOI] [PubMed] [Google Scholar]
- 3.Saposnik G., Noel de Tilly L., Caplan L.R. Pontine warning syndrome. Arch. Neurol. 2008;65 doi: 10.1001/archneur.65.10.1375. [DOI] [PubMed] [Google Scholar]
- 4.Tassi R., Cerase A., Acampa M., D’Andrea P., Guideri F., Lo Giudice G., Marotta G., Bracco S., Martini G. Stroke warning syndrome: 18 new cases. J. Neurol. Sci. 2013;331:168–171. doi: 10.1016/j.jns.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 5.Rahman Masum, Tadi Prasanna. Pons, Neuroanatomy. StatPearls Publishing; Treasure Island (FL): 2021. [PubMed] [Google Scholar]
- 6.Enriquez-Marulanda A., Amaya-Gonzalez P., Orozco J.L. Pontine warning syndrome: a Chameleon of ischemic stroke. Neurologist. 2016;21:93–96. doi: 10.1097/NRL.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 7.García-Esperón C., López-Cancio E., Martín-Aguilar L., Millán M., Castaño C., Munuera J., Dávalos A. Fluctuating locked-in syndrome as a presentation of a bilateral pontine infarction. NeuroRadiol. J. 2016;29:347–349. doi: 10.1177/1971400916658896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Algahtani H.A., Shirah B.H., Seddeq Y.A., Alshehri S.A. Unilateral headache and convulsive-like movements as a manifestation of pontine warning syndrome. Neurosciences. 2019;24:332–334. doi: 10.17712/nsj.2019.4.20190032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tahsili-Fahadan P., Simpkins A.N., Leigh R., Merino J.G. Stuttering lacunar infarction captured on serial MRIs. Neurol.: Clin. Pract. 2016;6:e37–e39. doi: 10.1212/CPJ.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naqvi I., Simpkins A.N., Cullison K., Elliott E., Reyes D., Leigh R., Lynch J.K. Recurrent thrombolysis of a stuttering lacunar infarction captured on serial MRIs. ENeurologicalSci. 2018;13:14–17. doi: 10.1016/j.ensci.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi G.M., Wang X.R., Xu W., Guo M.W., Ding C.Q., Zhou R.J. Pontine warning syndrome and restless legs syndrome secondary to paramedian pontine infarction: a case report. Int. J. Neurosci. 2020 doi: 10.1080/00207454.2020.1849187. [DOI] [PubMed] [Google Scholar]
- 12.Chan D.K., Silver F.L. Basilar artery stenosis mimicking the lacunar syndrome of pure motor hemiparesis. Can. J. Neurol. Sci. 2003;30:159–162. doi: 10.1017/s0317167100053452. [DOI] [PubMed] [Google Scholar]
- 13.Sen A., Birns J., Bhalla A. Stroke warning syndromes. Br. J. Hosp. Med. 2020;81:1–5. doi: 10.12968/hmed.2019.0222. [DOI] [PubMed] [Google Scholar]
- 14.Huang H.W., He S.W., Tan S.Q., Su L.L. Patient with pontine warning syndrome and bilateral posterior internuclear ophthalmoplegia: case report. BMC Neurol. 2010;10:2–5. doi: 10.1186/1471-2377-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadarajan V., Adesina T. Capsular warning syndrome. BMJ Case Rep. 2013:1–4. doi: 10.1136/bcr-2013-010503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delcker A., Diener H.C., Timmann D., Faustmann P. The role of vertebral and internal carotid artery disease in the pathogenesis of vertebrobasilar transient ischemic attacks. Eur. Arch. Psychiatr. Clin. Neurosci. 1993;242:179–183. doi: 10.1007/BF02189960. [DOI] [PubMed] [Google Scholar]
- 17.Katsanos A.H., Kosmidou M., Kyritsis A.P., Giannopoulos S. Is vertebral artery hypoplasia a predisposing factor for posterior circulation cerebral ischemic events? A comprehensive review. Eur. Neurol. 2013;70:78–83. doi: 10.1159/000351786. [DOI] [PubMed] [Google Scholar]
- 18.Katsanos A.H., Giannopoulos S. Increased risk for posterior circulation ischaemia in patients with vertebral artery hypoplasia: a systematic review and meta-analysis. Eur. Stroke J. 2017;2:171–177. doi: 10.1177/2396987317700540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulyk C., Voltan C., Simonetto M., Palmieri A., Farina F., Vodret F., Viaro F., Baracchini C. Vertebral artery hypoplasia: an innocent lamb or a disguise? J. Neurol. 2018;265:2346–2352. doi: 10.1007/s00415-018-9004-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.