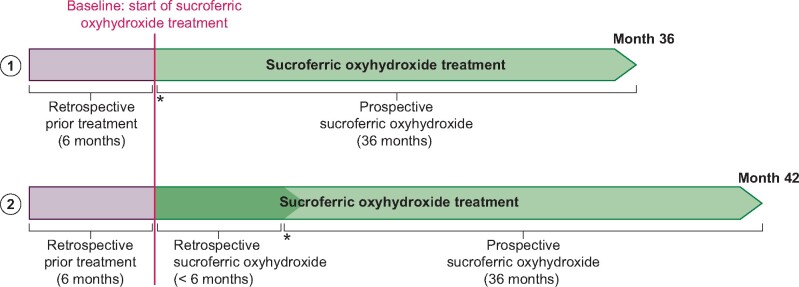
FIGURE 1:
VERIFIE study design. (1) Enrolment option for patients with SFOH treatment initiation at the time of inclusion into the study (treatment-naïve patients): treatment-naïve patients have a prospective observation period of up to 36 months. Retrospective data covering a period of 6 months prior to SFOH treatment start are collected. (2) Enrolment option for patients with SFOH treatment start up to 6 months prior to inclusion into the study (pre-treated patients): in pre-treated patients, SFOH treatment is documented for up to 42 months (for patients pre-treated with SFOH for >3 months and up to 6 months) including retrospective documentation of SFOH treatment up to 6 months and a prospective observation period of up to 36 months. Additionally, retrospective data covering a period of 6 months prior to SFOH treatment start are collected. Asterisks indicate inclusion into the study.