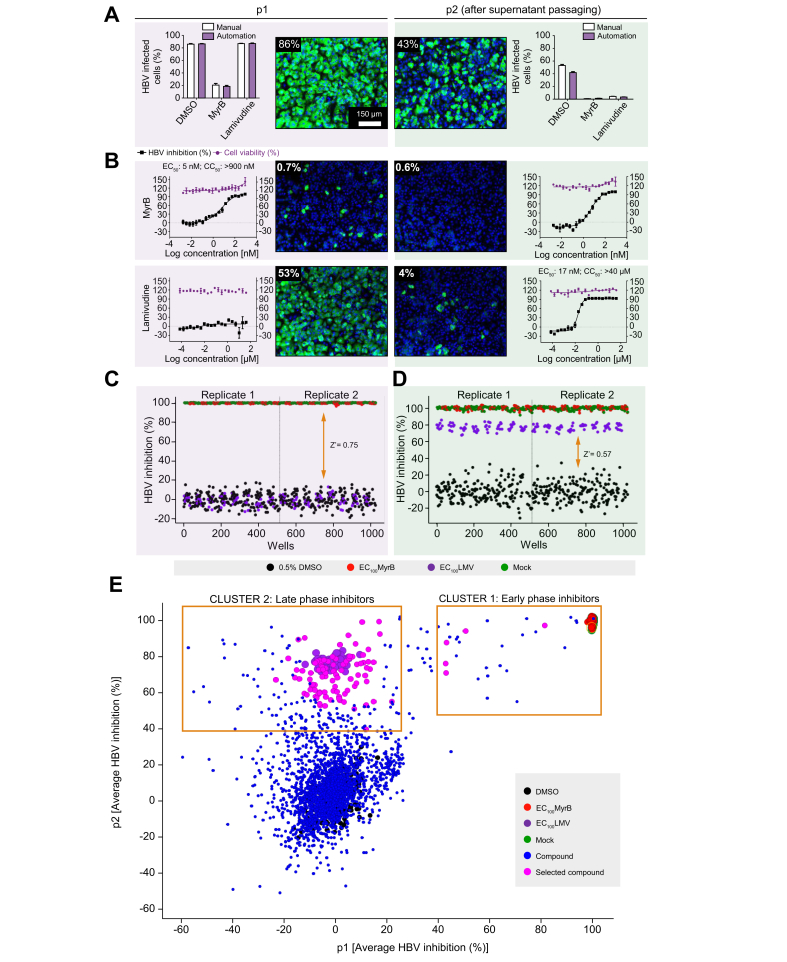
Fig. 3.
Adaptation of HBV infection assay for HTS automation.
The HBV 2-step infection SOP was adapted to laboratory automated liquid handling devices for HTS studies. (A) Comparison of HBV infection efficiency under manual dispensing vs. automated liquid handling conditions. DMSO- (0.5%, v/v), MyrB- (900 nM), or LMV-treated (40 μM) HepG2-NTCPsec+ p1 cells were inoculated with 2,000 GEq/cell HBV before the supernatants were transferred to naïve p2 cells at 6 dpi. A representative image is shown for infected p1 and p2 cells treated with 0.5% DMSO (v/v). (B) DRC analysis with the early HBV life cycle reference inhibitor MyrB (starting from 900 nM), and the late life cycle reference inhibitor LMV (starting from 40 μM) with 20-points 2-fold serial dilutions. Representative images are shown. HBV inhibition (black squares) and cell viability (red circles) are shown as percentages indicated by the left and right y-axes, respectively. Control performance in (C) p1 and (D) p2 cells. Scatterplot distribution shows HBV inhibition (%) with 0.5% DMSO (v/v) (black), 900 nM MyrB (red), 40 μM LMV (purple), and mock infection (green). (E) Compound distribution of average HBV inhibition (%) in p1 and p2 cells. Clusters of compounds exhibiting different activity patterns are depicted within yellow squares. CC50, 50% cytotoxic concentration; cccDNA, covalently closed circular DNA; dpi, days post-infection; DRC, dose–response curve; HTS, high-throughput screening; GEq, genome equivalents; LMV, lamivudine; MyrB, myrcludex B; PF-rcDNA, protein-free relaxed circular DNA; SOP, standard operation procedure.