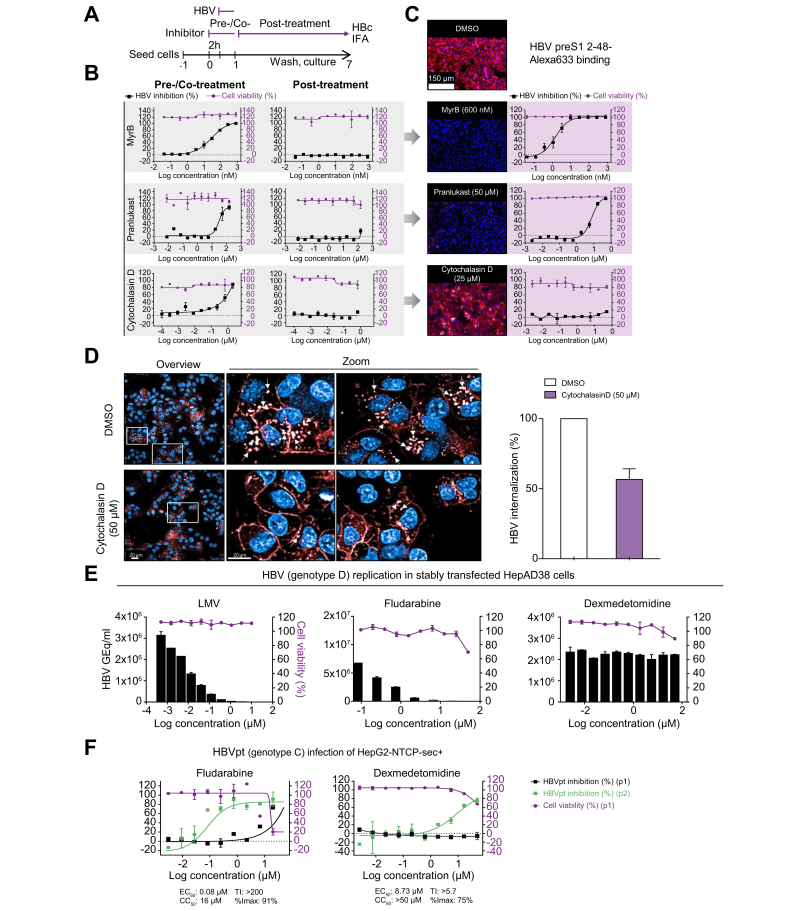
Fig. 5.
Mechanism of action studies with early and late HBV life cycle inhibitors.
(A) Time-of-addition experiment schedule. The reference inhibitors MyrB, pranlukast, and cytochalasin D were either pretreated and cotreated, or treated post-HBV inoculation in time-of-addition experiments. (B) DRC analysis of time-of-addition experiments. (C) Binding assay of HBV surface protein-derived Alexa633-labelled preS1 peptide under Mock (DMSO) or MyrB, pranlukast, cytochalasin D treatment. (D) Live-cell imaging of HBV surface protein-derived Alexa633-labelled preS1 peptide internalization (red) under cytochalasin D treatment. (E) Evaluation of HBV late step inhibitors in stably HBV replicating HepAD38 cells treated with serial dilutions of LMV, fludarabine, or dexmedetomidine. Supernatants were analysed by quantitative PCR at 7 days post-treatment. Cell viability was determined by automated nuclei counting. (F) Evaluation of late step inhibitors with HBVpt. HepG2-NTCPsec+ cells were pre-treated with 3-fold serially diluted fludarabine or dexmedetomidine (both starting from 50 μM) and infected with HBVpt genotype C. At 10 dpi, HBV inhibition of p1 cells (black squares) was analysed as described above, and supernatants were transferred to naïve p2 cells and analysed at 10 days post transfer (blue squares). Cell viability was determined as described before (red circles). Percentages of HBV inhibition and cell viability are indicated on the left and right y-axes, respectively. %Imax, percent maximum inhibition; CC50, 50% cytotoxic concentration; dpi, days post-infection; DRC, dose–response curve; GEq, genome equivalents; HBVpt, patient-derived HBV; IFA, immunofluorescence analysis; LMV, lamivudine; MyrB, myrcludex B; Ti, therapeutic index.