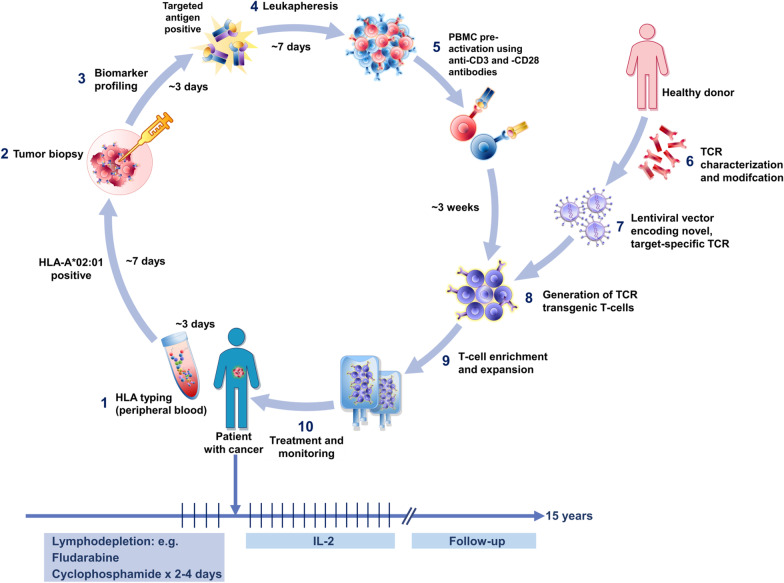
Fig. 1.
Schematic view of TCR-based adoptive T-cell therapy. (1) Patient’s screening starts with HLA typing. If HLA is A*02:01 type, a tumor biopsy is performed (2) to screen the tumor tissue for the expression of the targeted antigen (3), followed by leukapheresis (4). PBMCs from patient leukapheresis are isolated and pre-activated using anti-CD3 and -CD28 antibodies (5). A target-specific TCR is isolated from a healthy donor, characterized, and modified (6). A lentiviral vector is constructed and used to transfer the target-specific TCR in the T-cells (7). The activated PBMCs are transduced with a lentiviral vector encoding the target-specific TCR (8). Transduced T-cells are expanded to large numbers in 3–5 days and are frozen (9). Upon completion of the release testing, the T-cells are ready to be infused (10). Patients are typically treated with lymphodepletion, followed by T-cell product infusion, followed by low-dose interleukin 2. Patients are monitored for as long as 15 years to observe for delayed adverse events following exposure to the investigational gene therapy product