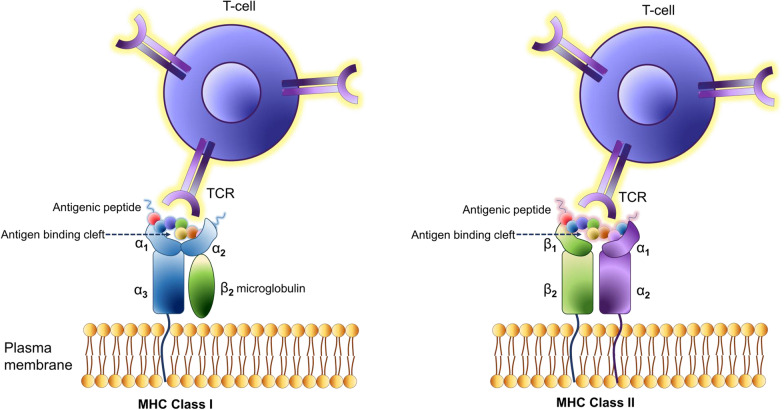
Fig. 2.
Schematic view of MHC class I and MHC class II molecules. MHC class I and class II molecules have high levels of polymorphism; a similar three-dimensional structure; a genetic location within one locus; and a similar function in presenting peptides to the immune system. MHC class I molecules present peptides at the cell surface to CD8 + T-cells, whereas MHC class II molecules present peptides to CD4 + T-cells that are derived from proteins degraded in the endocytic pathway. MHC class II molecules are primarily expressed by professional antigen-presenting cells (APCs), such as dendritic cells, macrophages, and B cells, and are conditionally expressed by other cell types. The transmembrane α- and β-chains of MHC class II molecules are assembled in the ER and associate with the invariant chain (Ii). The resulting Ii-MHC class II complex is transported to a late endosomal compartment termed the MHC class II compartment (MIIC). Here, the variant chain is digested, leaving a residual class II-associated Ii peptide (CLIP) in the peptide-binding groove of the MHC class II heterodimer. In the MIIC, MHC class II molecules require the chaperone HLA-DM to facilitate the exchange of the CLIP fragment for a specific peptide derived from a protein degraded in the endosomal pathway. MHC class II molecules are then transported to the plasma membrane to present their peptide cargo to CD4 + T-cells. In B cells, a modifier of HLA-DM is expressed called HLA-DO, and this protein associates with HLA-DM and restricts HLA-DM activity to more acidic compartments, thus modulating peptide binding to MHC class II molecules