Figure 5.
Imaging expansion gels
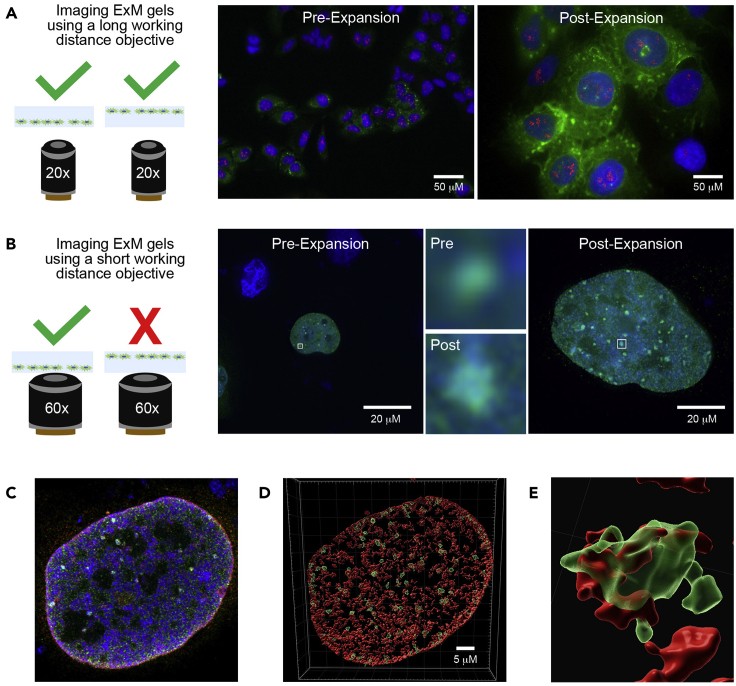
When using a lower magnification objective with a longer working distance, the orientation of the cells embedded in the gel with respect to the bottom of the well is not critical (A). The increase in size and observable detail is demonstrated here by imaging U2OS cells pre- and post-expansion using a Zeiss AxioObserver D1 widefield system with a 20×/0.8 NA air objective. In addition to the Hoechst-stained DNA (blue), the cells express GFP-G3BP2, boosted by staining with anti-GFP and AlexaFluor488 secondary antibodies (green), which has accumulated in cytoplasmic stress granules due to sodium arsenite treatment. Nucleoli have been stained using anti-fibrillarin (1:50 dilution) and AlexaFluor555 secondary antibodies (red). When using a higher magnification objective with a much shorter working distance, the cells embedded in the gel can only be brought into focus if they are lying directly on the bottom of the well (B). More detail can be observed for smaller structures (in this case, nuclear stress foci at which GFP-tagged RepoMan accumulates in response to sodium arsenite treatment) following expansion when a 60×/1.4 NA oil objective is used on a DeltaVision Core restoration deconvolution system. The white boxes mark the regions that have been enlarged to demonstrate the increased detail. Although this is already super-resolution imaging, we routinely combine ExM with Airyscan imaging on a Zeiss LSM880 laser confocal scanning system, which provides a further increase in resolution (C). Volume rendering of the 3D z-stacks using Imaris helps to visualize the nuclear substructure (D), confirming that nuclear stress foci (containing GFP-RepoMan, green) overlap and are surrounded by localized accumulations of the heterochromatic epigenetic histone marker H3K9me3 (1:50 dilution; red; E).