Fig. 4.
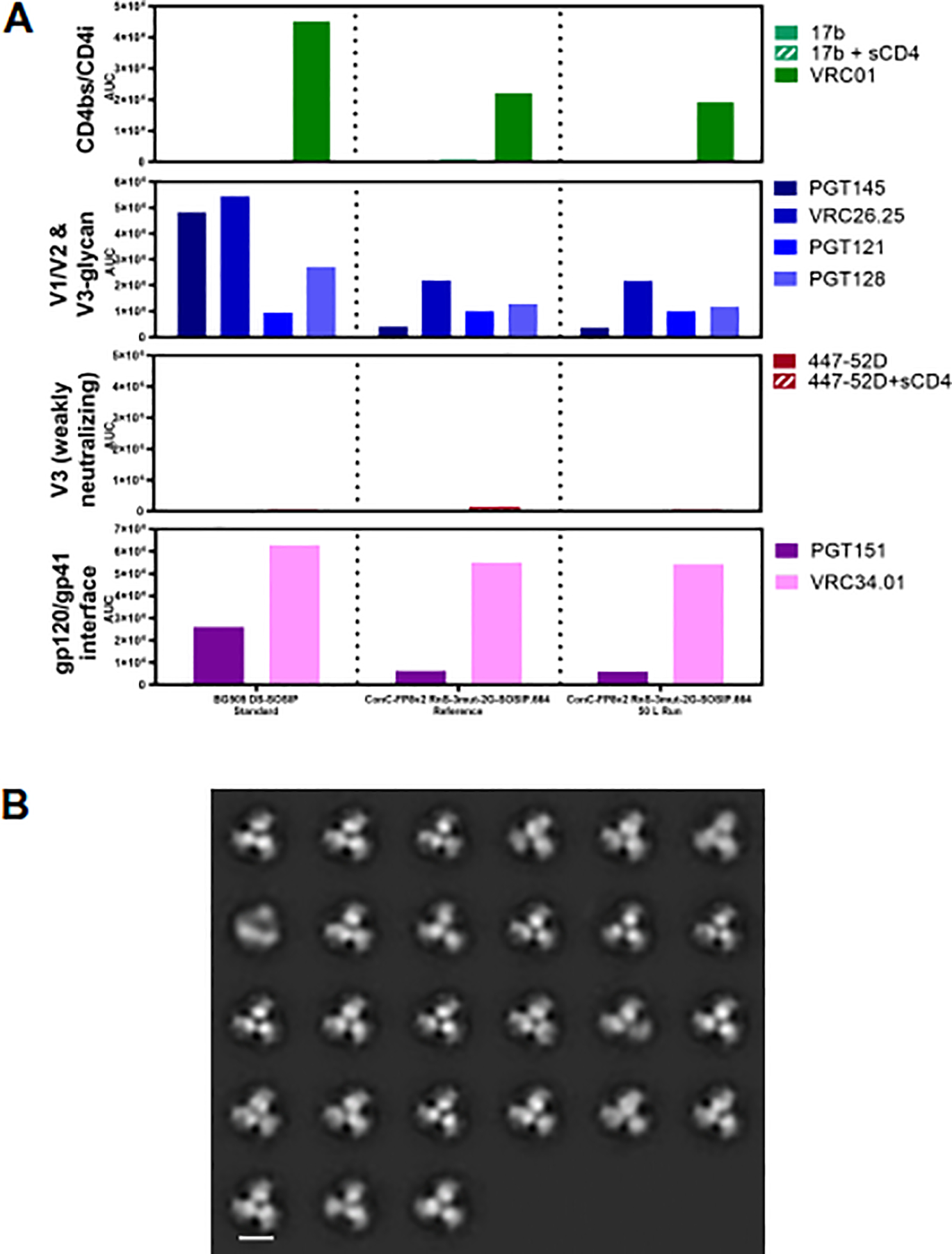
Antigenic and structural properties of ConC-FP8v2 RnS-3mut-2G-SOSlP.664 trimer. (A) Antigenicity, as determined by MSD, is shown in AUC for two development lots of ConC-FP8v2 RnS-3mut-2G-SOSlP.664 trimer and a BG505 DS-SOSIP standard. Antigenicity was assessed on a panel of antibodies, including broadly neutralizing antibodies directed against the CD4-binding site (VRC01), V1/V2-apex (PGT145 and CAP256-VRC2625), glycan-V3 site (PGT121 and PGT128)and fusion peptide epitope (VRC34.01 and PGT151). The panel also includes non/weakly neutralizing CD4-induced (17b) and V3-directed (447–52D) antibodies. (B) Negative stain electron microscopy class averages for purified ConC-FP8v2 RnS-3mut-2G-SOSIP.664 trimer from a 50 L scale production show correctly formed HIV-1 Env trimers in a prefusion-closed conformation. Scale bar corresponds to 10 nm.
