Abstract
Birth timing is a key life‐history characteristic that influences fitness and population performance. For migratory animals, however, appropriately timing birth on one seasonal range may be constrained by events occurring during other parts of the migratory cycle. We investigated how the use of capital and income resources may facilitate flexibility in reproductive phenology of migratory mule deer in western Wyoming, USA, over a 5‐yr period (2015–2019). Specifically, we examined how seasonal interactions affected three interrelated life‐history characteristics: fetal development, birth mass, and birth timing. Females in good nutritional condition at the onset of winter and those that migrated short distances had more developed fetuses (measured as fetal eye diameter in March). Variation in parturition date was explained largely by fetal development; however, there were up to 16 d of plasticity in expected birth date. Plasticity in expected birth date was shaped by income resources in the form of exposure to spring green‐up. Although individuals that experienced greater exposure to spring green‐up were able to advance expected birth date, being born early or late with respect to fetal development had no effect on birth mass of offspring. Furthermore, we investigated the trade‐offs migrating mule deer face by evaluating support for existing theory that predicts that births should be matched to local peaks in resource availability at the birth site. In contrast to this prediction, only long‐distance migrants that paced migration with the flush of spring green‐up, giving birth shortly after ending migration, were able to match birth with spring green‐up. Shorter‐distance migrants completed migration sooner and gave birth earlier, seemingly trading off more time for offspring to grow and develop over greater access to resources. Thus, movement tactic had profound downstream effects on birth timing. These findings highlight a need to reconsider classical theory on optimal birth timing, which has focused solely on conditions at the birth site.
Keywords: birth timing, capital–income breeding spectrum, carry‐over effects, full annual cycle ecology, green‐wave surfing, migration, mule deer, Odocoileus hemionus, seasonal interactions
Introduction
For animals living in seasonal environments, timing of birth is a key life‐history characteristic that influences offspring survival and population dynamics (Festa‐Bianchet 1988, Coulson et al. 2003). Natural selection should favor the alignment of energy‐expensive reproductive events with periods of resource abundance (Price et al. 1988, Williams et al. 2017). Additionally, birth should be timed to minimize risks associated with exposure of early newborns to late‐season storms (Descamps et al. 2015) or predation (Estes 1976), while assuring that young have time to grow large enough to survive periods of resource deficiency (Festa‐Bianchet 1988, Côté and Festa‐Bianchet 2001, Tomotani et al. 2016). Thus, existing theory suggests that local resource availability and seasonality at the birth site should shape optimal timing of birth (Peláez et al. 2020). When animals fail to time birth appropriately with local resource peaks, they often experience reduced fitness (Both et al. 2009, Harrison et al. 2011).
Migratory animals with complex life cycles face additional constraints that can influence birth timing. Specifically, key life‐history events (e.g., mating or moulting in birds) that occur on one seasonal range or during migration may constrain reproductive events that occur on another seasonal range (Both 2010, Tomotani et al. 2016, Tomotani et al. 2018). Such “seasonal interactions” occur when events in one part of the annual cycle have downstream effects at other times of the year, or vice versa (Marra et al. 2015). For example, when warmer temperatures facilitated earlier breeding in the migratory pied flycatcher (Ficedula hypoleuca), early‐born chicks were more likely to survive and successfully recruit as adults because they had more time to fatten up before moult and migration (Tomotani et al. 2016, 2018). Thus, time can be a limiting resource (Post 2019). Notably, the time needed to grow and develop before the onset of migration provides an alternative hypothesis, beyond local conditions at the birth site, of potentially important factors shaping birth timing in migratory species.
Migration itself may impose a time constraint on reproductive cycles as well, especially for long‐distance migrants (Both 2010). In spring, migrating birds often use a time‐minimizing tactic for spring migration because they must complete migration before establishing territories or finding mates on breeding ranges (Lindstrom and Alerstam 1992, Karlsson et al. 2012). Moreover, instead of using a purely time‐minimizing tactic, some migratory waterfowl and many migratory ungulates forage extensively during spring migration by pacing their migratory movements with the flush of young and highly nutritious plant green‐up that sweeps across the landscape along elevational or latitudinal gradients (Drent et al. 1978, Fryxell 1991, Merkle et al. 2016, Aikens et al. 2017). This phenomena, called “surfing the green wave,” allows migrants to increase access to high‐quality forage (Drent et al. 1978, Albon and Langvatn 1992, van der Graaf et al. 2006), which likely helps to finance reproduction (Parker et al. 2009). Consequently, for migratory ungulates and other taxa that forage extensively during migration, there may be a trade‐off between maximizing time for offspring growth and resource acquisition used to finance reproduction. Early births may provide sufficient time for offspring to grow before experiencing the physiological stresses of fall and winter resource scarcity. In contrast, late births may allow pregnant females more time to increase resource gain while surfing the green wave during migration. Migrating birds can assess conditions on the breeding range before initiating breeding. In contrast, migratory ungulates are constrained by long gestation periods (often >200 d). They mate in autumn on a distinct seasonal range far from the birth site and therefore breed without information on future conditions at the birth site (Loe et al. 2005, Mysterud et al. 2008, Ricklefs 2010). Understanding factors shaping reproductive phenology for migratory animals is contingent upon understanding constraints that occur outside of the range where birth occurs (Marra et al. 2015).
Although migrants face many constraints that shape the timing of reproductive events, physiological adaptations may allow for some degree of plasticity to either advance or delay reproductive events. Individuals can use resources derived from capital or income to finance the nutritional requirements of reproduction. A capital breeder relies on energy reserves stored within the body or in food caches, whereas an income breeder relies on currently available resources from the environment (Jönsson 1997). Capital and income breeding are often viewed as endpoints along a spectrum of potential tactics used to finance reproduction (Stephens et al. 2009). Reliance on capital or income resources can vary within and across species (Yohannes et al. 2010, Hogg et al. 2017). In ungulates, both experimental and wild studies suggest that gestation length is flexible (reviewed in Clements et al. 2011). For example, female bison (Bison bison) in good condition that bred late were able to advance gestation to synchronize birth with bison that bred earlier (Berger 1992). Although flexibility in gestation length has been attributed to factors related to both income and capital (Clements et al. 2011, Williams et al. 2017), the degree to which migratory ungulates use capital or income to fine‐tune birth timing is poorly understood (but see Holand et al. 2006).
Here, we investigate how constraints imposed by migration shape birth timing, and the degree to which the use of capital or income resources facilitates plasticity in birth date, in a population of mule deer (Odocoileus hemionus) in a mountainous region of western Wyoming, USA, 2015–2019 (Fig. 1). To examine how migratory tactic shapes reproductive phenology, we focus on three interrelated life‐history traits: fetal development, birth mass, and birth timing. Specifically, we evaluated how seasonal interactions arising from maternal condition (fat stores in December or March), migration tactic, and forage acquisition (green‐wave surfing during migration) shaped when animals gave birth and if birth matched local resource peaks.
Fig. 1.
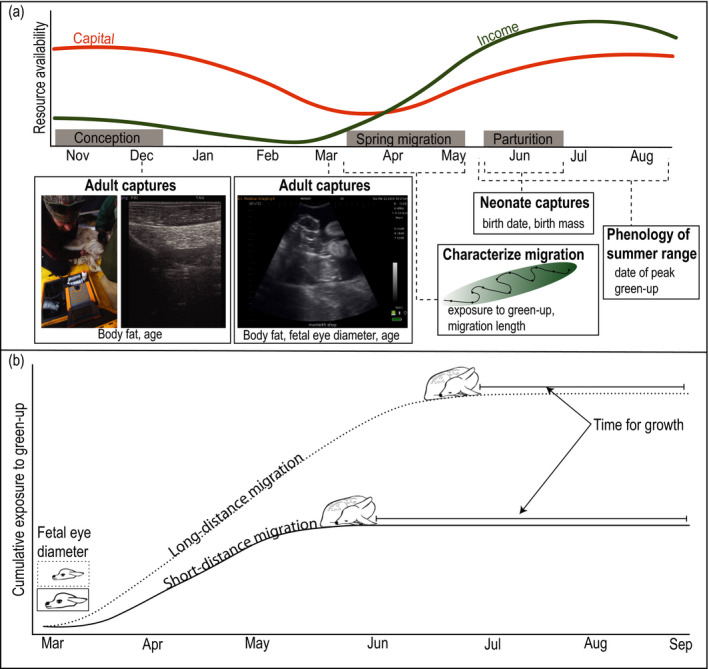
An overview of data collection, life‐history timeline and the predicted influence of migration distance on birth timing. (a) Timeline of life‐history events for migratory mule deer (Odocoileus hemionus) in western Wyoming, USA and data collection schedule during the study period (2015–2019). Within a year, there is variation in the availability of capital resources (body fat; orange line) along with seasonal fluctuations in income resources (high‐quality forage; green line). (b) A conceptual diagram illustrating how migration distance may shape birth timing.
For each life‐history trait we developed predictions that stem from the principles of resource allocation and annual‐cycle ecology (Marra et al. 2015). We expected that reliance on capital or income resources at various stages of the reproductive cycle would vary depending on their seasonal availability (Fig. 1a). We predicted that animals in better condition entering winter would have advanced fetal development (more capital investment) compared with animals entering winter in poorer condition. Furthermore, we predicted that long‐distance migrants would have delayed fetal development compared with shorter‐distance migrants, to avoid giving birth before reaching summer range (Fig. 1b). Next, we investigated if being born early or late influenced birth mass. We predicted that early‐born individuals would be smaller than late‐born individuals, because they had less time to grow and develop (Berger 1992, Karadaev et al. 2018). We also investigated if proxies of capital and income resource allocation could explain plasticity in birth date. We predicted that better maternal condition at the beginning of spring (capital) and higher exposure to spring green‐up (income) would result in earlier than expected birth, whereas poorer maternal condition and lower exposure to spring green‐up would delay parturition.
To test if migration constrains optimal birth timing, we assessed the classical assumption that animals should match offspring birth with peak resource availability at the birth site (Festa‐Bianchet 1988, Post et al. 2003). If conditions at the birth site are the sole driver of birth timing, we expected alignment between birth date and date of peak green‐up at the birth site. Alternatively, we considered a potential trade‐off between increased access to resources and increased time for offspring growth (Fig. 1b). Increased resources could be accrued through surfing the green wave, whereas increased time for offspring growth resulting from earlier birth would curtail opportunities to surf (Fig. 1b). If such a trade‐off exists, we expected only those animals that surf the green wave during migration, and give birth shortly after ending migration, to match birth with peak green‐up. At the other end of the spectrum, those individuals that maximize time for offspring to grow and develop are predicted to end migration sooner, allowing the green wave to pass by (Fig. 1b). In contrast to the trade‐off above highlighting how migration could constrain optimal birth timing, we also assessed the possibility that the need to give birth could constrain migration. Specifically, we explored if birth constrained green‐wave surfing by forcing deer to end migration early or causing them to overtake the green wave so they could reach summer range before giving birth (Bischof et al. 2012).
Methods
Study area
Our research took place in western Wyoming, USA (42°250 N, 110°420 W), a semiarid region in the Rocky Mountains. Mule deer in this system typically migrate 10–150 km from lower‐elevation (~1,800 m) winter ranges in the sage‐brush steppe to higher‐elevation (~2,300 to 2,750 m) summer ranges typified by a mixture of tall forb, aspen, mixed‐mountain shrub, and conifer communities (see Aikens et al. 2017 for details; Appendix S1: Fig. S1). Mating and conception occur in fall and early winter, either during fall migration or on winter ranges. Deer remain on winter ranges until early spring, when the emergence of green‐up triggers the start of spring migration (Monteith et al. 2011, Aikens et al. 2017). The timing of spring migration varies depending on migration distance and environmental variability (Sawyer et al. 2016, Aikens et al. 2020), but typically occurs between March and May. Birth typically occurs in June (Fig. 1a).
Adult capture
Each March and December from 2014 to 2019, we captured and recaptured a radiomarked group of 70, adult (>1 yr old), female mule deer using helicopter‐net guns (Barrett et al. 1982). During the study period, any mortality events that occurred within the group of monitored individuals were replaced with newly captured individuals during the following March or December capture period. To replace animals that died, we randomly selected an adult female from the same wintering area as the individual that had died. Newly captured animals were fit with a GPS collar, and recaptured animals received a GPS collar swap as needed given battery life and the fix schedule of the collar (fix rate varied from every 1 to 5 h, depending on collar manufacturer; see Aikens et al. 2017 for details). Details on the sample size and age of animals from each year of the study are in Appendix S1.
We used nutritional condition as a proxy for capital resource availability. During March and December captures, we estimated nutritional condition by combining measurements of body mass, depth of rump fat (measured via ultrasonography) and body‐condition scores, to calculate ingesta‐free body fat (IFBFat) using standardized protocols for mule deer (Stephenson et al. 2002, Cook et al. 2007, Cook et al. 2010; see Appendix 2 in Aikens et al. 2017 for details). Captures in December allowed us to quantify nutritional condition during the onset of winter, after autumn migration was completed (Fig. 1a). Captures in March of the following year quantified change in nutritional condition over winter, right before the initiation of spring migration (Aikens et al. 2020; Fig. 1a). We examined if age influenced body condition and found no relationship (Appendix S1; Fig. S2). In March, we collected additional data on pregnancy, fetal number, and stage of fetal development via ultrasonography (Karadaev et al. 2018; Appendix S2; Fig. 1a). We used fetal eye diameter as a proxy for stage of fetal development (Karadaev et al. 2018) and to examine plasticity in birth date given stage of fetal development. If a deer was pregnant (94.7% of all captured females), they were fit with a vaginal implant transmitter (VIT). We used the VITs that were expelled during a birth event to determine date of birth and to locate the birth site.
Neonate capture
During spring we monitored pregnant females each day to identify birthing events using VITs and movement behavior. Following a notification of VIT expulsion, or based on a combination of reduced step length and increased first‐passage time calculated daily during the birth season, we located females and confirmed if a birth event had occurred. We identified birth sites based on the presence of blood, hair, placenta, and the location of the VIT. After confirming presence of a birth site or birth event through visual observation of the adult female and location of the VIT, we searched the surrounding area for neonates. Upon capture of a neonate, we collected data on sex, mass, and morphometric measurements (Fig. 1a). We only included data from mother and offspring pairs in our analysis if the offspring was captured within 24 h of birth since birth mass was a critical response variable in our analyses (Monteith et al. 2014). All animal capture and handling protocols were approved by the Institutional Animal Care and Use Committee at the University of Wyoming (protocols 20131111KM00040, 20151204KM00135, 20170215KM00260).
Quantifying income and green‐wave surfing
We used remotely sensed data of the normalized difference vegetation index (NDVI) to quantify changes in plant phenology across space and time. We used the instantaneous rate of green‐up (IRG), to estimate exposure to spring green‐up and quantify green‐wave surfing behavior (see Appendix S3 for details). The IRG is scaled from 0 to 1, with a value of 1 representing the maximum exposure to spring green‐up for a given point on the landscape. From the IRG curves, we also calculated the date of peak green‐up as the Julian date of maximum IRG. Although remotely sensed data on plant phenology are coarse and subject to sources of noise, the date of peak green‐up derived from the methodology described above is strongly correlated with forage quality in the Greater Yellowstone Ecosystem (Geremia et al. 2019), where our study took place. We paired GPS collar data with IRG and date of peak green‐up data to quantify exposure to spring green‐up (IRG) and green‐wave surfing behavior (Days‐From‐Peak). Days‐From‐Peak is the absolute difference in days between the date of peak green‐up and the date of animal use, where a value of zero represents a perfect match between the date of peak green‐up and the date of animal use (Aikens et al. 2017). We used exposure to spring green‐up and date of peak green‐up on winter and summer range as proxies for the availability of income resources.
Additionally, we were interested in exploring if resource phenology on winter and summer range influenced timing of birth. The date of peak green‐up on winter and summer ranges represents the duration of resource availability across the landscape used by an individual. The date of peak green‐up on winter range represents when critical income resources first become available, whereas the date of peak green‐up on summer range represents peak forage quality at the birth site. For each female deer and year, we calculated date of peak green‐up on winter range as the average of the date of peak green‐up of all points that fell within a 1‐km circular buffer of the start of spring migration (a proxy of when spring green‐up first becomes available at low elevation). To quantify peak green‐up on summer range, we calculated average date of peak green‐up of all cells that fell within a 1‐km circular buffer of the birth‐site location (a proxy of when peak green‐up is available on summer range). To calculate if animals match birth with timing of peak resource quality, we calculated the difference, in days, between date of birth and date of peak green‐up at the birth site on summer range.
To examine if the need to give birth constrained green‐wave surfing, we calculated average Days‐From‐Peak for each individual day without taking the absolute difference, such that negative values represented birth before the green wave arrived, and positive values represented birth after the wave had passed by. We calculated individual and population‐level averages of Days‐From‐Peak for 60 d up to and including the date of birth in each year. We also calculated the number of days between the end of migration and the date of birth to quantify if birth constrained green‐wave surfing.
Migration timing and distance
To quantify migration timing, we used net squared displacement (NSD), which is the squared Euclidean distance between a reference point (usually the first GPS location in a year) and subsequent relocations, calculated for each animal year (Bunnefeld et al. 2011). For migratory animals, the NSD plotted across time has a distinctive shape similar to a double‐logistic curve (Bunnefeld et al. 2011). Specifically, movements on winter range at the beginning of the year correspond to relatively small NSD values. Spring migration movements away from winter range corresponds to a rapid increase in the NSD plotted through time. When migration is complete and movement is restricted to a summer range, an asymptote in NSD is reached. And finally, a decline in NSD corresponds to fall migration. Thus, times when there is a rapid increase or decrease in NSD can be used to identify the start and end of spring and fall migration events. We identified these migration events for each individual using annual NSD profiles, following Aikens et al. (2017). Then, we calculated migration distance as the Euclidean distance between the start and end of spring migration locations.
Statistical analysis
To measure plasticity in birth date given fetal development in March, we parameterized a linear model predicting the number of days to birth from the day the animal was handled (date range = 7 March–19 March) as a function of fetal eye diameter. The model of the number of days from capture to birth included fetal eye diameter, fetal number and year as fixed effects. We used residuals of this model to estimate plasticity in birth date, given fetal development, which we hereafter refer to as “plasticity in expected birth date.”
We developed three linear models to examine the factors influencing stage of fetal development, birth timing, and birth mass, respectively. For each of the three models, we included all predictor variables hypothesized to influence the metrics of interest and determined relative importance of variables based on statistical significance (α = 0.05) and effect size of the coefficient estimate. Specifically, we parameterized the model of stage of fetal development using migration distance and maternal nutritional condition (measured as IFBFat) in December. The model of birth timing included fetal eye diameter, to control for differences in stage of fetal development, exposure to spring green‐up (income), maternal nutritional condition in March (capital), date of peak green‐up on winter range (i.e., when income resources become available), and date of peak green‐up on summer range. The model of birth mass included the residuals from the plasticity model (to examine the role of being born early or late given fetal development), fetal number, exposure to spring green‐up, and maternal nutritional condition in March. To assess goodness of fit of the models, we used multiple R 2. We used the variance inflation factor (VIF) of each coefficient estimate to assess collinearity. If needed, we removed any predictor variables from the model with a VIF > 2, and then examined their relative influence on the response variable separately. We investigated the effect of repeated‐measures across GPS‐collared individuals tracked in multiple years and found that pseudoreplication did not bias our results (Appendix S4).
Results
Short migration distance and more capital resources resulted in advanced fetal development
A combination of IFBFat during the previous December and migration distance explained 22.3% of variance in fetal eye diameter (multiple R 2, degrees of freedom [df] = 47, variance inflation factor [VIF] = 1.00). A one‐unit increase in December IFBFat increased fetal eye diameter by 0.17 mm (equivalent to advancing birth by 0.47 d; P = 0.021), and a 10‐km increase in migration distance decreased fetal eye diameter by 0.23 mm (equivalent to delaying birth by 0.63 d; P = 0.008, Fig. 2).
Fig. 2.
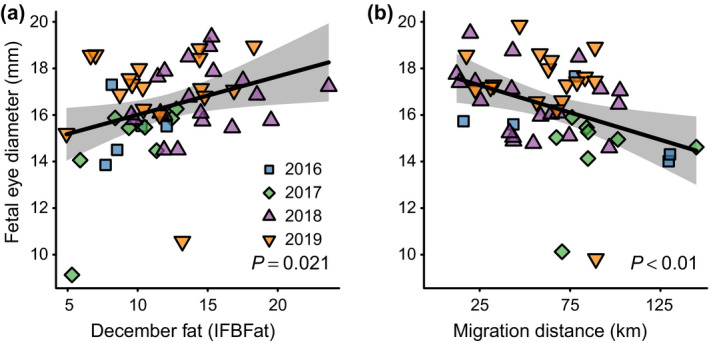
The influence of maternal condition (a) and migration distance (b) on late‐stage fetal development of mule deer in western Wyoming, USA, 2016–2019. Maternal condition was measured as ingesta‐free body fat (IFBFat) in December, whereas late‐stage fetal development was measured as fetal eye diameter in March. Both IFBFat and fetal eye diameter were measured using ultrasonography. The gray polygons represent the 95% confidence intervals for the predicted values (black line). The data points represent marginal effects at the mean for other covariates in the model.
Birth date was plastic, but plasticity did not influence birth mass
Timing of parturition varied across years (median dates of births: in 2015 = 8 June, in 2016 = 11 June, in 2017 = 19 June, in 2018 = 11 June, and in 2019 = 18 June), with 80% of births occurring within a range as short as 7.2 d in 2015 to as long as 21.5 d in 2017 (Fig. 3a). Fetal eye diameter, fetal number, and a fixed effect of year explained 72.2% of variance in birthdate (multiple R 2; df = 71, VIF = 1.57, 1.06, 1.65 for fetal eye diameter, fetal number and year, respectively). Residuals of expected birth date indicated that offspring could be born anywhere from 16 d earlier to 10 d later than expected based on late‐stage fetal development. Being born early or late with respect to fetal development had no effect on birth mass, nor did exposure to spring green‐up, maternal condition in March, or fetal number (P > 0.1 for all coefficient estimates, df = 73).
Fig. 3.
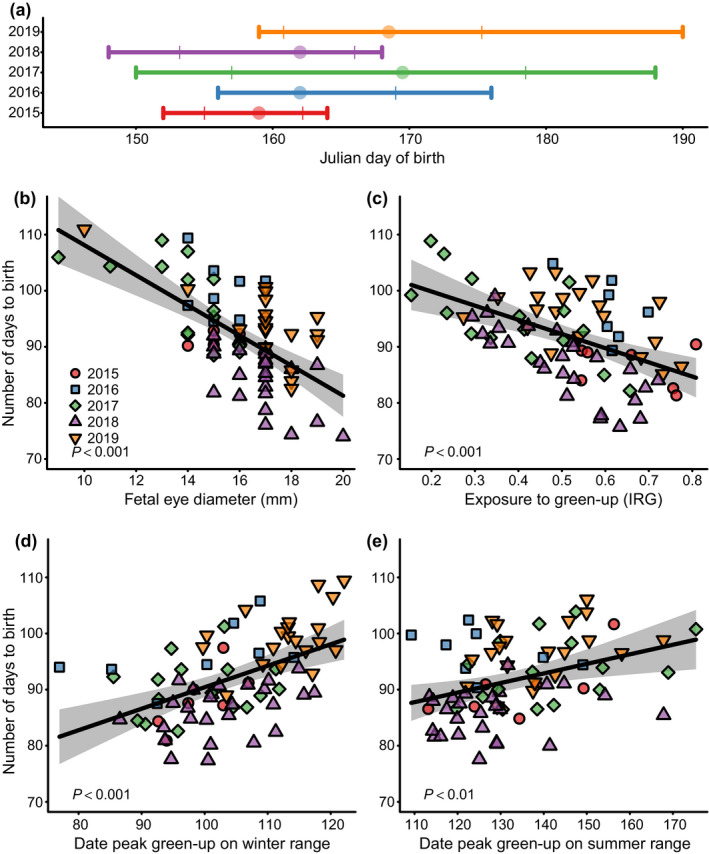
Factors affecting parturition date of mule deer in western Wyoming, USA, 2015–2019. (a) The distribution of parturition dates in 2015 (red), 2016 (blue), 2017 (green), 2018 (purple), and 2019 (orange), with the whiskers representing the full range of parturition dates, thin lines representing when 80% of births occurred, and circles representing the median parturition date of each year. (b) The relationship between number of days to birth (i.e., expected birth date) and fetal eye diameter. (c) The relationship between exposure to spring green‐up and the number of days until birth. Exposure to spring green‐up was measured as the instantaneous rate of green‐up (IRG), with a value of 1 representing maximum exposure to spring green‐up. (d) The influence of the date of peak green‐up on winter range and (e) summer range on expected birthdate. Date of peak green‐up on winter range represents when income resources become available at low elevation and the date of peak green‐up on summer range marks when peak resource quality occurs at the birth site. The gray polygons are the 95% confidence intervals for the predicted values (black lines) in (b–e). The data points in (b–e) represent marginal effects at the mean for other covariates in the model.
Increased availability of income resources advanced expected birth date
Expected birth date was explained largely by late‐stage fetal development, exposure to spring green‐up, and date of peak green‐up on winter and summer ranges (multiple R 2 = 0.645, df = 70). A millimeter increase in fetal eye diameter corresponded to a 2.69‐d advancement in birthdate (P < 0.0001, VIF = 1.18; Fig. 3b), whereas a 0.1 unit increase in IRG advanced birth date by 2.53 d (P < 0.0001, VIF = 1.27; Fig. 3c). Likewise, a day delay in peak green‐up on winter range and summer range corresponded to a 0.38‐ and a 0.17‐d delay in expected birthdate respectively (P < 0.01, VIF = 1.24 [winter range] and 1.35 [summer range]; Fig. 3d, e). Fetal number and maternal condition (i.e., IFBFat in March) had no effect on expected birthdate (P > 0.1).
Trade‐off between time for offspring growth and access to resources was mediated by migration distance
Most animals completed migration well before giving birth. Across the 5‐yr period of this study, animals completed migration on average 23 d before giving birth (mean in 2015 = 22 d, in 2016 = 23.5 d, in 2017 = 18.5 d, in 2018 = 25.2 d, in 2019 = 22.5 d). There was individual variability, however, in the number of days between completion of migration and birth, with three birth events (3.85%) occurring 1 d before migration was completed (Fig. 4a). Animals that ended migration earlier also gave birth earlier (r 2 = 0.15, P < 0.001, β = 0.19). The date of peak green‐up on summer range was a strong predictor of the end of migration (r 2 = 0.41, P < 0.001; Fig. 4b), with each day delay in date of peak green‐up at summer range corresponding to a 0.53‐d later completion of migration. Likewise, there was individual variation in the mismatch between the date of peak green‐up on summer range and the date of birth. Animals born earlier were more mismatched with peak green‐up in comparison with animals born later (r 2 = 0.12, P < 0.01, β = 0.20; Fig. 4c). The degree to which birth was mismatched with peak green‐up on summer range was linked to green‐wave surfing (Days‐From‐Peak), with animals that surfed closer to peak green‐up during migration giving birth more synchronously with peak green‐up (Fig. 4d). Specifically, every day closer to peak green‐up that animals surfed during migration resulted in a 1.88‐d closer match between birth and peak green‐up on summer range (r 2 = 0.37, P < 0.001). Date of peak green‐up across individual summer ranges differed by a minimum of 40 d (in 2016) to a maximum of 56 d (in 2017). On average, animals tended to track the green wave closely in early spring (±15 d of peak green‐up; Fig. 4e). But once migration was completed, animals quickly became decoupled from the green wave—a pattern that was consistent across all 5 yr (Fig. 4e). Thus, the need to give birth did not constrain green‐wave surfing, as most animals finished migration before giving birth (Fig. 4).
Fig. 4.
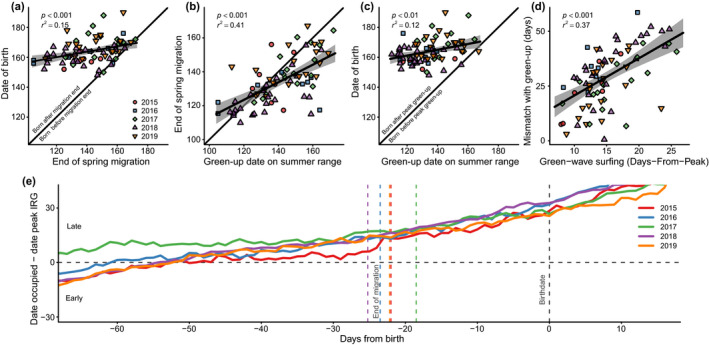
Factors affecting the match between peak green‐up on summer range and parturition of mule deer in western Wyoming, USA. (a) The relationship between end of spring migration and date of parturition. (b) Relationship between date of peak green‐up on summer range and date that animals completed spring migration. (c) Relationship between date of peak green‐up on summer range and parturition date. The black 1:1 line represents the hypothetical expectation that timing of one event in the migratory life cycle is perfectly matched with timing of another event. (d) Relationship between green‐wave surfing and number of days between parturition and peak green‐up. Green‐wave surfing is measured as Days‐From‐Peak, where a value of zero represents perfect surfing. (e) Whether animals were early or late with respect to the green wave during a 60‐d period before birth (gray dashed line at 0 on the x‐axis). Positive values on the y‐axis represent animals being late (i.e., the wave has passed them by), and negative values represent being early (i.e., the wave has not yet arrived). Each colored line (red = 2015, blue = 2016, green = 2017, purple = 2018, orange = 2019) represents the population average in green‐wave surfing through time for that year. The vertical dashed lines in (e) represent the mean end of migration from 2015–2019, with each color representing the mean end of migration for that year.
Discussion
Via long‐term, individual‐based monitoring of movement behavior and timing of birth in a long‐lived ungulate, we revealed strong links among reproductive phenology, migratory tactic, and resource allocation. As predicted, the importance of capital or income resources depended on their seasonal availability and varied with stage of reproduction. Specifically, better maternal condition caused fetal development to be advanced when measured in March (Fig. 2), and availability of forage during migration advanced expected birth date (Fig. 3). Although fetal development was a strong predictor of expected birth date, there was up to 16 d of plasticity in birthdate beyond that predicted by fetal eye diameter. In contrast to our prediction, deer that advanced parturition date in response to better exposure to income resources in spring did so without compromising birth mass. In contrast to existing theory, which predicts that conditions at the birth site should shape optimal birth timing, our results provide a clear example of birth timing being shaped by trade‐offs arising from events occurring away from the birth site and from other parts of the annual cycle. Only animals that surfed the green wave and ended migration just before giving birth matched birth with peak green‐up, whereas most gave birth after peak green‐up. Although matching birth with peak green‐up likely increased access to high‐quality resources, doing so resulted in delayed birth and therefore, less time for offspring to grow and develop before fall migration. Together, our results suggest seasonal interactions arising from differences in migration distance and resource allocation shape birth timing. Integrating full annual cycle ecology into the study of birth timing highlights how seemingly distinct events occurring on spatially separated seasonal ranges and migratory routes are strongly intertwined.
In ungulates, timing of birth is shaped by two key factors: date of conception and gestation length. Both date of conception and gestation length are flexible and influenced by capital‐ and income‐based processes (Berger 1992, Langvatn et al. 2004, Clements et al. 2011). Indeed, mule deer in our study used resources derived from both capital and income to finance reproduction, but their use depended on the stage of fetal development. Maternal condition in December was important in timing of conception and early fetal development (Fig. 2), whereas factors related to income, including exposure to spring green‐up and the beginning of spring green‐up on winter and summer ranges, had a stronger effect later in fetal development (Fig. 3). Variation in conception date is shaped by estrus cycles of females, which can be influenced by nutritional condition, age, resource availability, density, and the presence of males (McComb 1987, Langvatn et al. 2004, Tyler et al. 2020). Gestation length has likewise been linked to maternal condition, age, and environmental conditions (Mysterud et al. 2009, Clements et al. 2011). Here, the reliance on income or capital resources for reproduction depended on seasonal resource availability. Capital was more important in early stages of reproduction when resource availability was low. Income was more important during late‐stage gestation in spring and early summer when better forage resources were available (Fig. 1a).
Birth timing did not always align with the commonly held assumption that birth should be matched with peak forage quality (Post et al. 2003, Williams et al. 2017). Animals that experienced greater exposure to spring green‐up were able to advance expected birthdate by 2.5 d for each 0.1 unit increase in IRG, compared with those that did not track vegetation phenology as well. Moreover, an earlier onset of spring green‐up on winter and summer range was associated with an advance in parturition date, and individuals that better matched parturition with peak green‐up more closely surfed the green wave. Although synchronizing birth may help to reduce predation on vulnerable young through predator swamping (Estes 1976), birth synchrony also is assumed to be the result of animals timing parturition to match peak resource availability (Post et al. 2003) or be in sync with long‐term climate averages (Bowyer et al. 1998). Nevertheless, individuals and populations vary in their timing of birth (Peláez et al. 2020), with animals born later often experiencing the greatest mismatch with resource availability (Festa‐Bianchet 1988). Likewise, our study revealed that in most instances animals did not match parturition with peak green‐up. But in contrast to previous work (Festa‐Bianchet 1988, Côté and Festa‐Bianchet 2001, Plard et al. 2014), animals born later did not experience more of a mismatch with resource peaks than those born early. Instead, deer that experienced a large mismatch between birth and peak green‐up tended to give birth earlier than individuals that achieved greater synchronization between parturition and peak green‐up (Fig. 4). Early birth often is linked to better offspring survival in ungulates, because early‐born animals have more time to grow before the onset of winter (Festa‐Bianchet 1988, Côté and Festa‐Bianchet 2001). The highly heterogeneous green‐up across summer ranges in our study might provide a unique challenge to balance early birth date, completion of migration, and synchronizing birth with peak green‐up. Indeed, animals that gave birth early tended to end migration early; however, the green wave had already passed them by long before birth. Thus, in this system animals appear to trade off early birth and increased time for offspring growth with matching birth to peak green‐up (Fig. 1b). Consequently, time, and not just forage availability, is a limiting resource for migrants (Harrison et al. 2011). Conceptualizing birth timing through the lens of the full annual cycle helps to illuminate additional trade‐offs that migrants face when balancing reproduction with migration, foraging, and accumulation of fat reserves.
In line with previous research (Verme 1965, Berger 1992, Asher et al. 2005), maternal condition was important in shaping conception date and gestation length. Nevertheless, the effect of migration distance on reproductive phenology is much less studied. The negative relationship between migration distance and fetal development indicates that movement tactic may manifest in varied life‐history strategies. Because animals that migrated long distances had less developed fetuses in March, long‐distance migrants must either mate later or have prolonged gestational development. Long‐distance migrants likely benefit from having less developed fetuses in March, thereby allowing them to complete migration before giving birth without sacrificing the ability to surf the green wave along their migratory route. In contrast, individuals that ended migration early also gave birth early, and thus may sacrifice an extended period of exposure to spring green‐up but benefit from additional time for their offspring to grow and develop before the onset of winter (Fig. 1b). Indeed, the effect of migration distance on fetal development indicates that resource allocation and life‐history strategy may be fine‐tuned to the movement tactic of an animal. Similarly, movement tactic of pectoral sandpipers (Calidris melanotos) also shaped resource allocation. Early‐arriving birds that migrated short distances relied on capital resources gained at staging areas to finance reproduction because resource availability is low early in the breeding season (Yohannes et al. 2010). In contrast, sandpipers that arrived late because they migrated long distances used readily available resources from the breeding range to finance reproduction (Yohannes et al. 2010). Studies investigating how movement tactic shapes reproductive phenology and resource allocation tactics within a species are rare (Monteith et al. 2014), despite providing a promising area for future research that may help to facilitate the link between individual differences in movement and fitness (Nathan et al. 2008).
The link between movement tactic and the timing of birth has important conservation and management implications. First, adjusting to anthropogenic disturbances that curtail migration may not be as simple as moving to undisturbed areas. The behavioral and physiological mechanisms that match movement with optimal reproductive phenology in a given environment also must change in tandem with altered movement behavior. Relocation efforts by wildlife management agencies show that it often takes several years for animals translocated from different ecotypes to adjust the timing of reproduction to the relocated site (Whiting et al. 2011, 2012). So, for animals that must drastically alter movement behaviors to cope with anthropogenic disturbances, a similar lag in physiological adjustments that negatively affect fitness and population performance also may occur. Second, populations with greater life‐history diversity are more robust in the face of environmental change or stochasticity—a phenomenon referred to as the portfolio effect (Schindler et al. 2010). The diversity of movement behaviors in our system represents more than differences in behavior—it represents diversity in life history that warrants conservation attention. Protecting and conserving movement diversity is beneficial to maintain populations that will be more resilient to environmental change, whereas the loss of movement diversity will make populations more vulnerable to local extinction (Schindler et al. 2010).
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Acknowledgments
Comments from D. Morris, A. Mysterud, M. Dillon, and two anonymous reviewers helped to improve earlier versions of this manuscript. Jerod A. Merkle provided the remotely sensed phenology layers. EOA was supported by a National Science Foundation Graduate Research Fellowship Program. The Wyoming Range Mule Deer project is supported by Wyoming Game and Fish Department, Wyoming Game and Fish Commission, Bureau of Land Management, Muley Fanatic Foundation (including Southwest, Kemmerer, Upper Green, and Blue Ridge Chapters), Boone and Crockett Club, Wyoming Wildlife and Natural Resources Trust, Knobloch Family Foundation, Wyoming Animal Damage Management Board, Wyoming Governor's Big Game License Coalition, Bowhunters of Wyoming, Wyoming Outfitters and Guides Association, Pope and Young Club, U.S. Forest Service, and U.S. Fish and Wildlife Service. We thank the multiple landowners who kindly offered access on their property for this research. We thank Brittany Wagler, Rachel Smiley, Ashleigh Rhea, Lindsay Clontz, Megan Ahern, Hannah Haeussler, Russell B. Allen, Juan Camacho, Spender Norland, Emily Monfort, Emily Moberg, Erin Wood, Brian Miller, Missy Stallard, Addison Trower, Saranda Oestericher, Tracey Faber, and Melinda Nelson for assistance with data collection. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The authors declare no competing interests. EOA, SPHD, TNL, MJK, and KLM conceived of the ideas and designed methodology. Cartography was by SPHD and the conceptual figure by RPJ. TNL, SPHD, RPJ, GLF, JR, RK, MT, and KLM collected the data. EOA analyzed the data and led the writing of the manuscript. All authors provided feedback on drafts.
Aikens E. O., Dwinnell S. P. H., LaSharr T. N., Jakopak R. P., Fralick G. L., Randall J., Kaiser R., Thonhoff M., Kauffman M. J., and Monteith K. L.. 2021. Migration distance and maternal resource allocation determine timing of birth in a large herbivore. Ecology 102(6):e03334. 10.1002/ecy.3334
Corresponding Editor: Douglas W. Morris.
Literature Cited
- Aikens, E. O. , Kauffman M. J., Merkle J. A., Dwinnell S. P. H., Fralick G. L., and Monteith K. L.. 2017. The greenscape shapes surfing of resource waves in a large migratory herbivore. Ecology Letters 20:741–750. [DOI] [PubMed] [Google Scholar]
- Aikens, E. O. , Monteith K. L., Merkle J. A., Dwinnell S. P. H., Fralick G. L., and Kauffman M. J.. 2020. Drought reshuffles plant phenology and reduces the foraging benefit of green‐wave surfing for a migratory ungulate. Global Change Biology 26:4215–4225. [DOI] [PubMed] [Google Scholar]
- Albon, S. D. , and Langvatn R.. 1992. Plant phenology and the benefits of migration in a temperate ungulate. Oikos 65:502–513. [Google Scholar]
- Asher, G. W. , Scott I. C., O’Neill K. T., and Littlejohn R. P.. 2005. Influence of level of nutrition during late pregnancy on reproductive productivity of red deer: (2) Adult hinds gestating wapiti × red deer crossbred calves. Animal Reproduction Science 86:285–296. [DOI] [PubMed] [Google Scholar]
- Barrett, M. W. , Nolan J., and Roy L. D.. 1982. Evaluation of a hand‐held net‐gun to capture large mammals. Wildlife Society Bulletin 10:108–114. [Google Scholar]
- Berger, J. 1992. Facilitation of reproductive synchrony by gestation adjustment in gregarious mammals: a new hypothesis. Ecology 73:323–329. [Google Scholar]
- Bischof, R. , Loe L. E., Meisingset E. L., Zimmermann B., Van Moorter B., and Mysterud A.. 2012. A migratory northern ungulate in the pursuit of spring: Jumping or surfing the green wave? American Naturalist 180:407–424. [DOI] [PubMed] [Google Scholar]
- Both, C. 2010. Flexibility of timing of avian migration to climate change masked by environmental constraints en route. Current Biology 20:243–248. [DOI] [PubMed] [Google Scholar]
- Both, C. , Van Asch M., Bijlsma R. G., Van Den Burg A. B., and Visser M. E.. 2009. Climate change and unequal phenological changes across four trophic levels: constraints or adaptations? Journal of Animal Ecology 78:73–83. [DOI] [PubMed] [Google Scholar]
- Bowyer, R. T. , Van Ballenberghe V., and Kie J. G.. 1998. Timing and synchrony of parturition in Alaskan moose: long‐term versus proximal effects of climate. Journal of Mammalogy 79:1332–1344. [Google Scholar]
- Bunnefeld, N. , Borger L., van Moorter B., Rolandsen C. M., Dettki H., Solberg E. J., and Ericsson G.. 2011. A model‐driven approach to quantify migration patterns: individual, regional and yearly differences. Journal of Animal Ecology 80:466–476. [DOI] [PubMed] [Google Scholar]
- Clements, M. N. , Clutton‐Brock T. H., Albon S. D., Pemberton J. M., and Kruuk L. E. B.. 2011. Gestation length variation in a wild ungulate. Functional Ecology 25:691–703. [Google Scholar]
- Cook, R. C. , et al. 2010. Revisions of rump fat and body scoring indices for deer, elk, and moose. Journal of Wildlife Management 74:880–896. [Google Scholar]
- Cook, R. C. , Stephenson T. R., Myers W. L., Cook J. G., and Shipley L. A.. 2007. Validating predictive models of nutritional condition for mule deer. Journal of Wildlife Management 71:1934–1943. [Google Scholar]
- Côté, S. D. , and Festa‐Bianchet M.. 2001. Birthdate, mass and survival in mountain goat kids: effects of maternal characteristics and forage quality. Oecologia 127:230–238. [DOI] [PubMed] [Google Scholar]
- Coulson, T. , Kruuk L. E. B., Tavecchia G., Pemberton J. M., and Clutton‐Brock T. H.. 2003. Estimating selection on neonatal traits in red deer using elasticity path analysis. Evolution 57:2879–2892. [DOI] [PubMed] [Google Scholar]
- Descamps, S. , Tarroux A., Varpe Ø., Yoccoz N. G., Tveraa T., and Lorentsen S.‐H.. 2015. Demographic effects of extreme weather events: snow storms, breeding success, and population growth rate in a long‐lived Antarctic seabird. Ecology and Evolution 5:314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drent, R. , Ebbinge B., and Weijand B.. 1978. Balancing the energy budgets of arctic‐breeding geese throughout the annual cycle: a progress report. Verhandlungen der Ornithologischen Gesellschaft in Bayern 23:239–264. [Google Scholar]
- Estes, R. D. 1976. The significance of breeding synchrony in the wildebeest. African Journal of Ecology 14:135–152. [Google Scholar]
- Festa‐Bianchet, M. 1988. Birthdate and survival in bighorn lambs (Ovis canadensis). Journal of Zoology 214:653–661. [Google Scholar]
- Fryxell, J. M. 1991. Forage quality and aggregation by large herbivores. American Naturalist 138:478–498. [Google Scholar]
- Geremia, C. , Merkle J. A., Eacker D. R., Wallen R. L., White P. J., Hebblewhite M., and Kauffman M. J.. 2019. Migrating bison engineer the green wave. Proceedings of the National Academy of Sciences of the United States of America 116:25707–25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, X. A. , Blount J. D., Inger R., Norris D. R., and Bearhop S.. 2011. Carry‐over effects as drivers of fitness differences in animals. Journal of Animal Ecology 80:4–18. [DOI] [PubMed] [Google Scholar]
- Hogg, J. T. , Dunn S. J., Poissant J., Pelletier F., and Byers J. A.. 2017. Capital vs. income‐dependent optimal birth date in two North American ungulates. Ecosphere 8:e01766. [Google Scholar]
- Holand, Ø. , Mysterud A., Røed K. H., Coulson T., Gjøstein H., Weladji R. B., and Nieminen M.. 2006. Adaptive adjustment of offspring sex ratio and maternal reproductive effort in an iteroparous mammal. Proceedings of the Royal Society B 273:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson, K. I. 1997. Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78:57–66. [Google Scholar]
- Karadaev, M. , Fasulkov I., Yotov S., Atanasova S., and Vasilev N.. 2018. Determination of the gestational age through ultrasound measurements of some uterine and foetal parameters in Bulgarian local goats. Reproduction in Domestic Animals 53:1456–1465. [DOI] [PubMed] [Google Scholar]
- Karlsson, H. , Nilsson C., Bäckman J., and Alerstam T.. 2012. Nocturnal passerine migrants fly faster in spring than in autumn: a test of the time minimization hypothesis. Animal Behaviour 83:87–93. [Google Scholar]
- Langvatn, R. , Mysterud A., Stenseth N. C., and Yoccoz N. G.. 2004. Timing and synchrony of ovulation in red deer constrained by short northern summers. American Naturalist 163:763–772. [DOI] [PubMed] [Google Scholar]
- Lindstrom, A. , and Alerstam T.. 1992. Optimal fat loads in migrating birds: a test of the time‐minimization hypothesis. American Naturalist 140:477–491. [DOI] [PubMed] [Google Scholar]
- Loe, L. , Bonenfant C., Mysterud A., Gaillard J. M., Langvatn R., Klein F., Calenge C., Ergon T., Pettorelli N., and Stenseth N.. 2005. Climate predictability and breeding phenology in red deer: timing and synchrony of rutting and calving in Norway and France. Journal of Animal Ecology 74:579–588. [Google Scholar]
- Marra, P. P. , Cohen E. B., Loss S. R., Rutter J. E., and Tonra C. M.. 2015. A call for full annual cycle research in animal ecology. Biology Letters 11:20150552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComb, K. 1987. Roaring by red deer stags advances the date of oestrus in hinds. Nature 330:648–649. [DOI] [PubMed] [Google Scholar]
- Merkle, J. A. , Monteith K. L., Aikens E. O., Hayes M. M., Hersey K. R., Middleton A. D., Oates B. A., Sawyer H., Scurlock B. M., and Kauffman M. J.. 2016. Large herbivores surf waves of green‐up during spring. Proceedings of the Royal Society B 283:20160456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteith, K. L. , Bleich V. C., Stephenson T. R., Pierce B. M., Conner M. M., Kie J. G., and Bowyer R. T.. 2014. Life‐history characteristics of mule deer: Effects of nutrition in a variable environment. Wildlife Monographs 186:1–62. [Google Scholar]
- Monteith, K. L. , Bleich V. C., Stephenson T. R., Pierce B. M., Conner M. M., Klaver R. W., and Bowyer R. T.. 2011. Timing of seasonal migration in mule deer: effects of climate, plant phenology, and life‐history characteristics. Ecosphere 2:art47. [Google Scholar]
- Mysterud, A. , Bonenfant C., Loe L. E., Langvatn R., Yoccoz N. G., and Stenseth N. C.. 2008. The timing of male reproductive effort relative to female ovulation in a capital breeder. Journal of Animal Ecology 77:469–477. [DOI] [PubMed] [Google Scholar]
- Mysterud, A. , Røed K. H., Holand Ø., Yoccoz N. G., and Nieminen M.. 2009. Age‐related gestation length adjustment in a large iteroparous mammal at northern latitude. Journal of Animal Ecology 78:1002–1006. [DOI] [PubMed] [Google Scholar]
- Nathan, R. , Getz W. M., Revilla E., Holyoak M., Kadmon R., Saltz D., and Smouse P. E.. 2008. A movement ecology paradigm for unifying organismal movement research. Proceedings of the National Academy of Sciences of the United States of America 105:19052–19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, K. L. , Barboza P. S., and Gillingham M. P.. 2009. Nutrition integrates environmental responses of ungulates. Functional Ecology 23:57–69. [Google Scholar]
- Peláez, M. , Gaillard J.‐M., Bollmann K., Heurich M., and Rehnus M.. 2020. Large scale variation in birth timing and synchrony of a large herbivore along the latitudinal and altitudinal gradients. Journal of Animal Ecology 89:1906–1917. [DOI] [PubMed] [Google Scholar]
- Plard, F. , Gaillard J.‐M., Coulson T., Hewison A. J. M., Delorme D., Warnant C., and Bonenfant C.. 2014. Mismatch between birth date and vegetation phenology slows the demography of roe deer. PLoS Biology 12:e1001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post, E. 2019. Time in ecology: a theoretical framework. Princeton University Press, Princeton, New Jersey, USA. [Google Scholar]
- Post, E. , Bøving P. S., Pedersen C., and MacArthur M. A.. 2003. Synchrony between caribou calving and plant phenology in depredated and non‐depredated populations. Canadian Journal of Zoology 81:1709–1714. [Google Scholar]
- Price, T. , Kirkpatrick M., and Arnold S.. 1988. Directional selection and the evolution of breeding date in birds. Science 240:798–799. [DOI] [PubMed] [Google Scholar]
- Ricklefs, R. E. 2010. Embryo growth rates in birds and mammals. Functional Ecology 24:588–596. [Google Scholar]
- Sawyer, H. , Middleton A. D., Hayes M. M., Kauffman M. J., and Monteith K. L.. 2016. The extra mile: Ungulate migration distance alters the use of seasonal range and exposure to anthropogenic risk. Ecosphere 7:e01534. [Google Scholar]
- Schindler, D. E. , Hilborn R., Chasco B., Boatright C. P., Quinn T. P., Rogers L. A., and Webster M. S.. 2010. Population diversity and the portfolio effect in an exploited species. Nature 465:609. [DOI] [PubMed] [Google Scholar]
- Stephens, P. A. , Boyd I. L., McNamara J. M., and Houston A. I.. 2009. Capital breeding and income breeding: their meaning, measurement, and worth. Ecology 90:2057–2067. [DOI] [PubMed] [Google Scholar]
- Stephenson, T. R. , Bleich V. C., Pierce B. M., and Mulcahy G. P.. 2002. Validation of mule deer body composition using in vivo and postmortem indices of nutritional condition. Wildlife Society Bulletin 30:557–564. [Google Scholar]
- Tomotani, B. M. , Gienapp P., Beersma D. G. M., and Visser M. E.. 2016. Climate change relaxes the time constraints for late‐born offspring in a long‐distance migrant. Proceedings of the Royal Society B 283:20161366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomotani, B. M. , van der Jeugd H., Gienapp P., de la Hera I., Pilzecker J., Teichmann C., and Visser M. E.. 2018. Climate change leads to differential shifts in the timing of annual cycle stages in a migratory bird. Global Change Biology 24:823–835. [DOI] [PubMed] [Google Scholar]
- Tyler, N. J. , Gregorini P., Parker K. L., and Hazlerigg D. G.. 2020. Animal responses to environmental variation: physiological mechanisms in ecological models of performance in deer (Cervidae). Animal Production Science 60:1248–1270. [Google Scholar]
- van der Graaf, S. A. J. , Stahl J., Klimkowska A., Bakker J. P., and Drent R. H.. 2006. Surfing on a green wave—How plant growth drives spring migration in the Barnacle Goose Branta leucopsis . Ardea 94:567–577. [Google Scholar]
- Verme, L. J. 1965. Reproduction studies on penned white‐tailed deer. Journal of Wildlife Management 29:74–79. [Google Scholar]
- Whiting, J. C. , Bowyer R. T., Flinders J. T., and Eggett D. L.. 2011. Reintroduced bighorn sheep: fitness consequences of adjusting parturition to local environments. Journal of Mammalogy 92:213–220. [Google Scholar]
- Whiting, J. C. , Olson D. D., Shannon J. M., Bowyer R. T., Klaver R. W., and Flinders J. T.. 2012. Timing and synchrony of births in bighorn sheep: implications for reintroduction and conservation. Wildlife Research 39:565–572. [Google Scholar]
- Williams, C. T. , Klaassen M., Barnes B. M., Buck C. L., Arnold W., Giroud S., Vetter S. G., and Ruf T.. 2017. Seasonal reproductive tactics: annual timing and the capital‐to‐income breeder continuum. Philosophical Transactions of the Royal Society B 372:20160250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yohannes, E. , Valcu M., Lee R. W., and Kempenaers B.. 2010. Resource use for reproduction depends on spring arrival time and wintering area in an arctic breeding shorebird. Journal of Avian Biology 41:580–590. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4


